2018 was a bad year for influenza. In the United States alone, 80,000 people died and nearly one million people were hospitalized as a result of the virus, according to the Center for Disease Control (CDC). It was the worse year since the last pandemic occurred in 2009.
While 2019 is thus far appearing to be less severe, the impact of the flu still remains large.
“We are calling the current season relatively mild, but even a mild season causes a significant number of illnesses, medical visits,” said Nancy Messonnier, MD, the Director of the National Center for Immunization and Respiratory Diseases (NCIRD) at the CDC. “There have been 200,000 flu hospitalizations so far in 2019 and more importantly 19,000 flu deaths, and this counts as a mild year. I think it’s really important that we keep that in perspective, because this is what we are looking to prevent.”
In addition to its severe impact on human health, the flu also has significant economic costs. According to Messonnier, the direct medical cost of the flu every year is almost 10 billion dollars.
“If you take into account the indirect cost—that is moms who have to stay home with their kids—it is closer to 87 billion dollars,” said Messonnier. “This is just seasonal flu, let’s imagine the potential impact of another pandemic.”
Messonnier recently spoke on the topic of flu vaccines at the American Association for the Advancement of Science (AAAS) Annual Meeting, held Feb. 14-17 in Washington D.C., as part of the scientific session, “The Quest for the Universal Flu Vaccine,” along with Anthony Fauci, MD, the Director of the National Institute of Allergy and Infectious Diseases Chief, Laboratory of Immunoregulation, Chief, Immunopathogenesis Section at the National Institutes of Health (NIH); and Gary Nabel, MD, PhD, the Chief Scientific Officer and Head of North America Research & Development Hub at Sanofi Genzyme.
An imperfect vaccine
The seasonal flu vaccine has been key to preventing wide spread flu infection since it was first approved in 1945. However, despite its benefits, the vaccine is far from perfect.
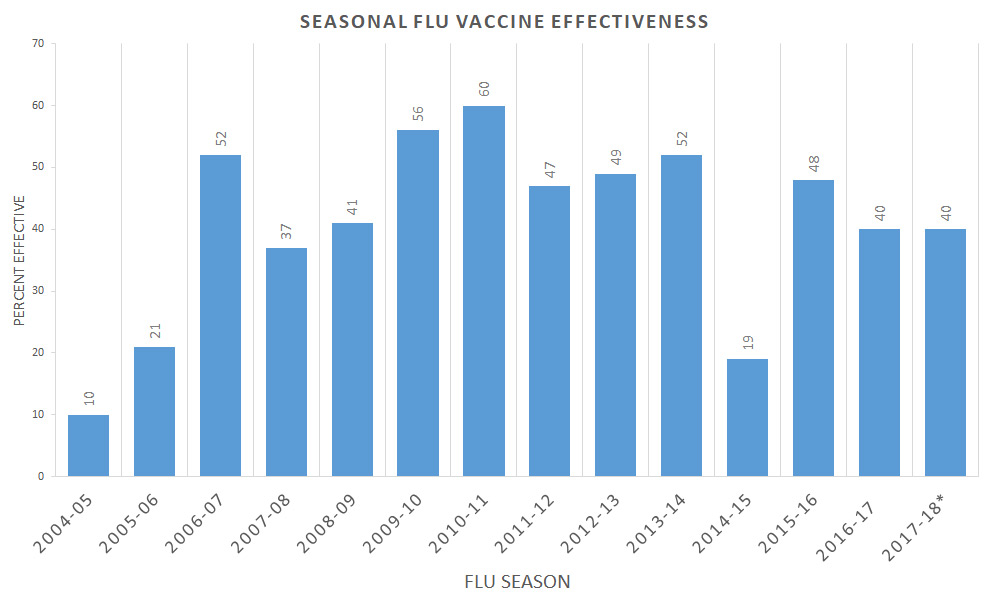
“Current influenza vaccines, although they have extraordinary value at avoiding morbidity and mortality, are none the less, not consistently effective,” said Fauci, during the AAAS scientific session. “This year, thus far we have about 47 percent efficacy, last year—one of the worst years that we’ve had in many years—the overall efficacy was about 40 percent and the efficacy against H3N2, the predominant strain, was only 25 percent. Clearly we all agree we need to do better.”
In the last 15 years, the highest flu vaccine efficacy reported was 60 percent during the 2010-2011 season. Efficacy dropped as low as 10 percent in 2004, according to the CDC.
During the presentation at the AAAS Annual Meeting, Messonnier noted that while these numbers are discouraging, it is important to remember that even low vaccine efficacy makes a big difference.
“Even a small amount of vaccine effectiveness can save lots of lives and prevent lots of hospitalizations,” she said.
Credit: CDC
A complex virus
Creating a flu vaccine is no easy task. The virus is extremely complex and is constantly mutating to avoid host immunity. There are 18 different subtypes of Influenza A and two subtypes of Influenza B, and each subtype has many, many strains.
“There are literally thousands of strains that we know of,” said Nabel, during the AAAS Annual Meeting scientific session. “This is one of the reasons that it is such a challenge to create a vaccine against a genetically diverse and biochemically diverse set of targets.”
Selecting which strains to target each year is one of the CDC’s biggest challenges.
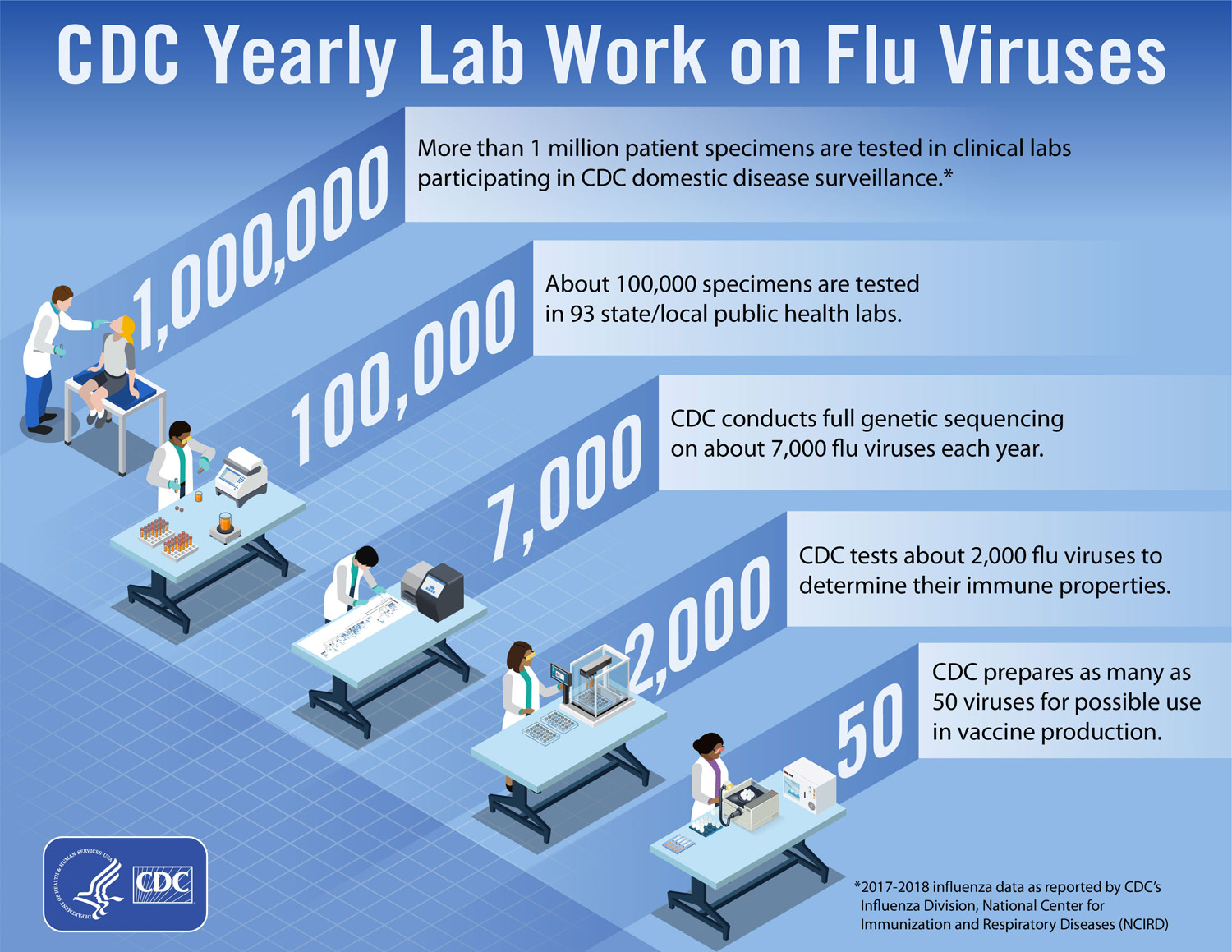
The CDC looks at which strains of the flu are circulating outside of the U.S. and tests over one million patient specimens in clinical labs within the U.S. every year. They then prepare as many as 50 viruses for possible use in vaccine production. The strains that will be included in that year’s seasonal flu vaccine must be selected in the spring in order to be ready for production by the fall, as the current process of growing most flu vaccines takes six months.
Credit: CDC
This long timeline is sometimes the cause of lower vaccine efficacy, said Fauci.
“You can have an antigenic mismatch, which is a well-known case of decreased influenza seasonal vaccines,” said Fauci. “In other words, you make your choice in February or March about what virus is going to be in the vaccine, but in the six or seven month period that you are growing it, there is a possibility that in nature, it will mutate and while you are making a vaccine for A, the virus turns to B.”
The most common way that flu vaccines are made is using an egg-based manufacturing process that has been used for more than 70 years.
In this process, the CDC, or one of their laboratory partners, provides pharmaceutical companies with candidate vaccine viruses grown in eggs. These virus are then injected into fertilized hen’s eggs and incubated for several days to allow the viruses to replicate. Eventually, the virus-containing fluid is harvested from the eggs and the influenza viruses are inactivated, purified and tested for use in the vaccine.
There are some strains of the flu that present challenges within egg-based manufacturing, particularly H3N2. These viruses are difficult to propagate in eggs, and sometimes, the virus even changes during the process.
Egg-based manufacturing is particularly problematic during a pandemic.
The last pandemic began in March 2009, when, at the end of the flu season, a new strain emerged in California and Mexico. By April it had spread to the rest of the world.
The strain was isolated right away, but because it takes six months to grow a virus in eggs, the vaccine would not be ready until October. Still, officials felt confident that, because viruses tend to peak in December and January, that the vaccine would be ready in time to treat a pandemic, said Fauci.
“We felt reasonably sure that by the time this inevitable pandemic came we would have doses,” he said. “However, in September 2009 we had the beginning of the pandemic as soon as the children came back to school. The cases began to peak in the early fall, and we didn’t get our vaccine doses until the late fall. So what we had was a vaccine that was far too late to be of substantial help.”
Making improvements on seasonal vaccines
While approximately 70 percent of flu vaccines are still made using eggs, other methods are coming to the forefront.
In 2016, the FDA issued an approval for Seqirus, the first cell-based flu vaccine manufacturer in the U.S., to use cell-grown vaccine viruses. In this method, the viruses are cultured into mammalian cells and allowed to replicate for a few days. Then, the virus-containing fluid is collected from the cells and the virus antigen is purified.
However, this approach still has limitations, said Fauci.
“I, and many of my colleagues, think that this still is not good enough,” said Fauci. “What we really need to do is get to the point where you never have to grow a virus to make a vaccine. You clone it, you put it in whatever vector you want to put it in, so you don’t need to wait for a seven month process to grow it.”
Strides toward this goal have been achieved. The FDA approved the first recombinant influenza vaccine, Flublok Quadrivalent, which is manufactured by Sanofi Pasteur, in 2013.
In this production method, manufacturers isolate a certain gene known as the hemagglutinin (HA) gene from a naturally occurring “wild type” recommended vaccine virus. This HA gene is then combined with portions of another virus that grows well in insect cells. This recombinant vaccine virus is then mixed with insect cells and allowed to replicate in these cells. The flu HA protein is then harvested from these cells and purified.
The quest for a universal vaccine
While it is critical that research continues that is focused on improving current seasonal vaccines and preventing pandemics, the ultimate goal should be to create a universal flu vaccine that protects patients against multiple strains of flu.
This won’t happen all at once, but in stages, said Fauci,
“To achieve a universal flu vaccine it is going to be a multiple step process, it’s not going to happen overnight, and we aren’t going to get to total universality right from the beginning. In fact we might not ever get to total universality.”
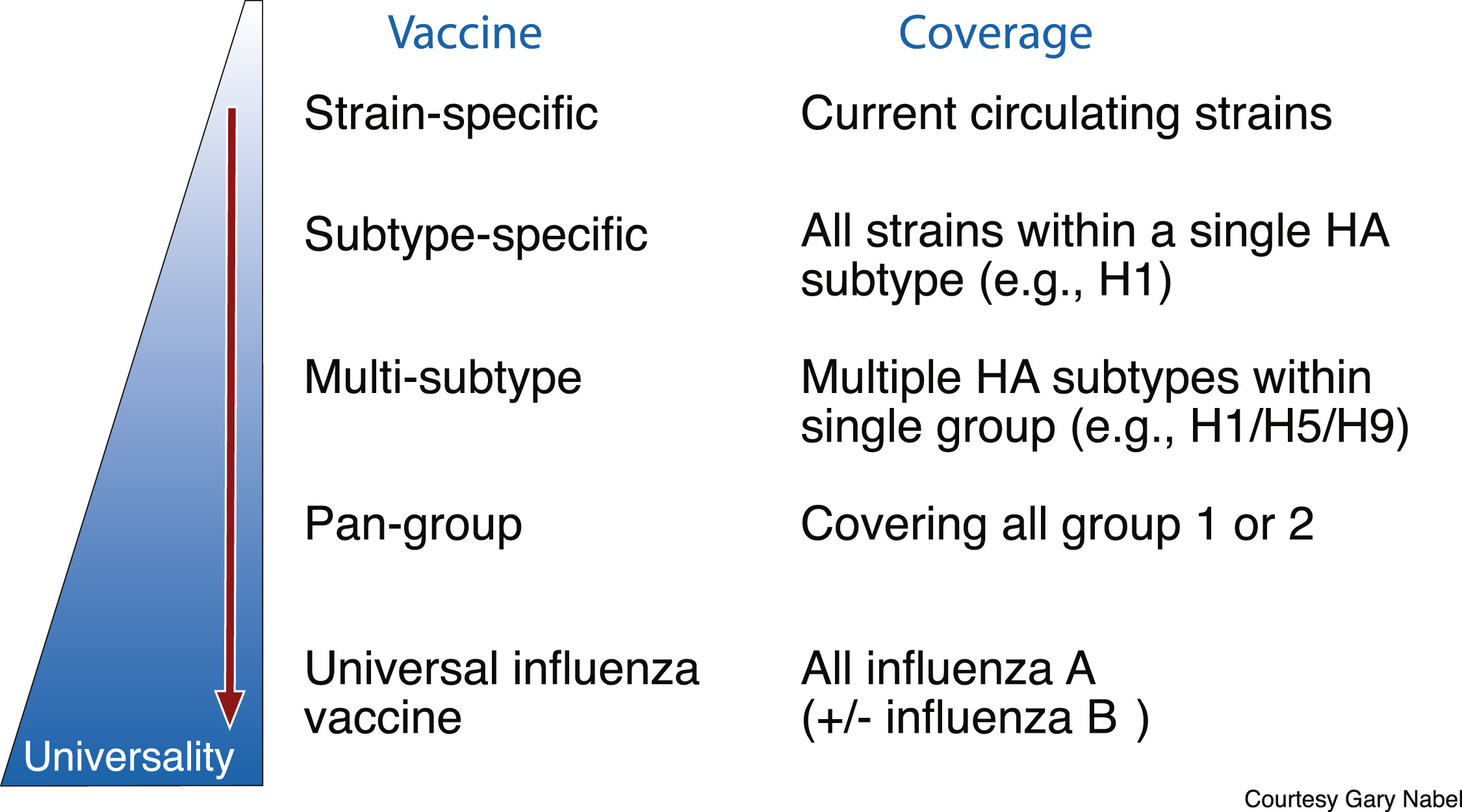
Steps towards a universal flu vaccine. Credit: Published by Oxford University Press for the Infectious Diseases Society of America, 2018
Current flu vaccines are strain-specific—designed to cover the most common strains that are circulating. The next step is to move to a subtype specific vaccine that targets all strains within a single HA subtype, such as all strains that are H1. Then the next goal would be to create a vaccine than targets multiple HA subtypes within a single group, then multiple groups, and then finally a vaccine that covers all influenza viruses.
Fauci and his colleagues at NIAID unveiled their strategic plan to make this happen, which was published in The Journal of Infectious Diseases in July 2018.
To develop a universal influenza vaccine, the NIAID plan states that the organization will focus resources on three key areas of influenza research: improving the understanding of the transmission, natural history and pathogenesis of influenza infection; precisely characterizing how protective influenza immunity occurs and how to tailor vaccination responses to achieve it; and supporting the rational design of universal influenza vaccines, including designing new immunogens and adjuvants to boost immunity and extend the duration of protection.
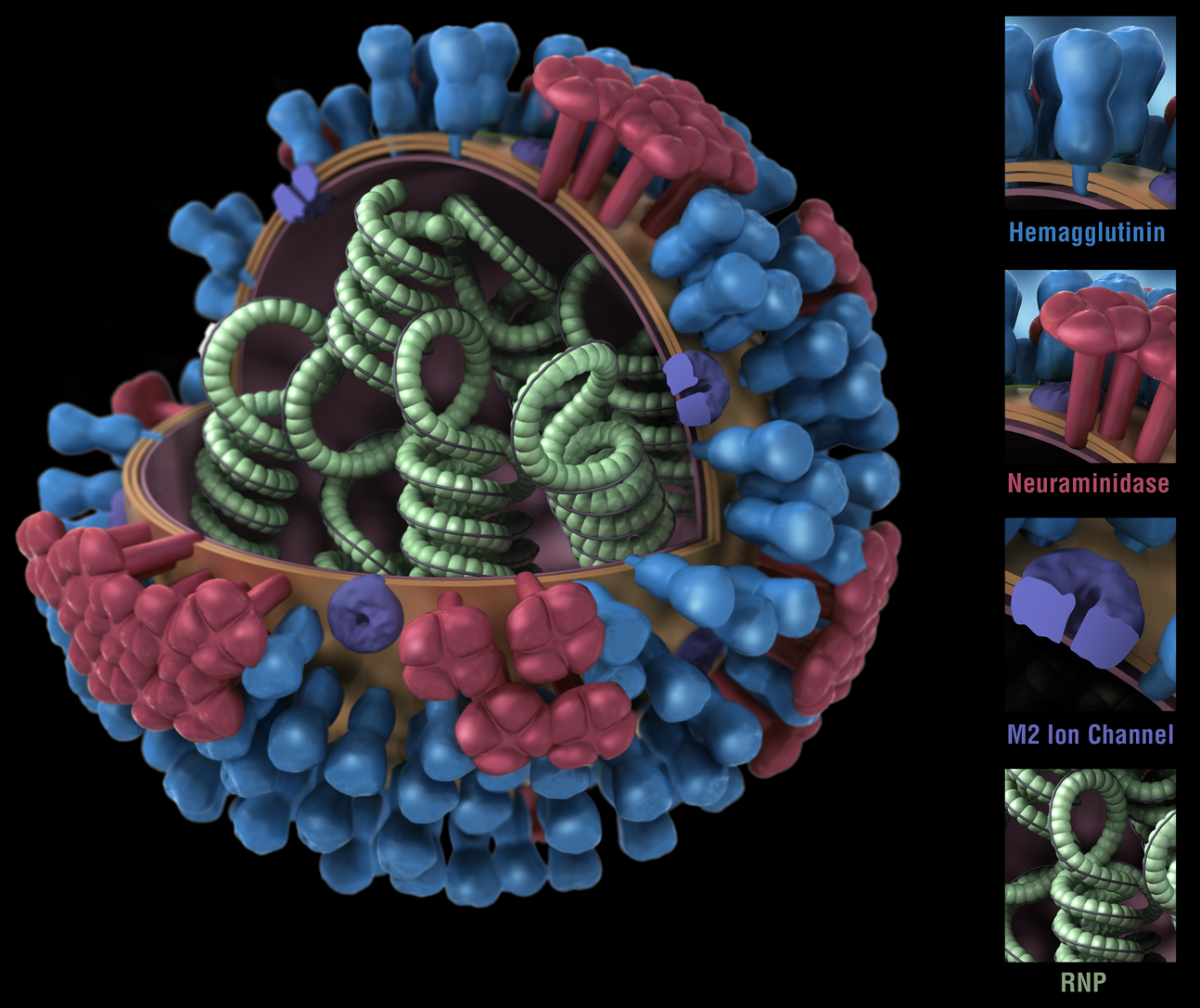
The above image shows different features of an influenza virus, including the surface proteins hemagglutinin (HA) and neuraminidase (NA). Credit CDC
Leading universal vaccine strategies
HA is likely a major target for universal flu vaccines, explained Fauci. Influenza has two surface glycoproteins: HA, which helps the virus enter host cells, and neuraminidase, which helps the virus spread among cells. HA is commonly likened to a mushroom because it has a round head and a stalk.
“When you look at the structural biology of the hemagglutinin, in the head region it is a highly mutational tending area,” said Fauci. “This is a major target for protection, which is good news, but the bad news is that it is highly specific for each strain, because it mutates readily. Whereas the stem region does not do that much at all. In other words, this is the part of hemagglutinin that does not change much at all from season to season. So the obvious approach is, why not direct the immune response to that part of the virus that doesn’t change from season to season from strain to strain, and perhaps even from pandemic to pandemic?”
A number of research organizations are investigating this approach. At the Icahn School of Medicine at Mount Sinai, flu microbiologist, Peter Palese, PhD, is working to create “chimeric” viruses where the HA head comes from bird flu and the stem from common human flu viruses.
To create a chimeric vaccine, the HA head is removed from the stalk of a currently circulating virus and swapped for the head of a virus that doesn’t typically infect humans. Unfamiliar heads are used because the body won’t have existing antibodies the vaccine can build upon, which decreases the immunodominance of the head region. Researchers hope this will force a stronger immune response against the stalk region, while the head region is relatively ignored and prompt an immune response against the portion of the virus that is similar across strains and across seasons—doing away with the need for annual vaccination.
The Chimeric HA approach is currently being tested in a phase I trial in partnership with pharmaceutical company GlaxoSmithKline, as well as NIAD.
NIAID is also studying various strategies to create a vaccine that elicits antibodies targeting the HA stem. NIAID scientists designed an experimental vaccine featuring the protein ferritin, which can self-assemble into microscopic nanoparticles. The vaccine showed promise in animal testing and is being evaluated for future trials in humans.
NIAID scientists also developed a vaccine incorporating four subtypes of the H protein into one vaccine. The vaccine is made from non-infectious virus-like particles that stimulate an immune response but cannot replicate or cause disease. Results have been promising in animal studies and may advance to human trials.
Phase 1/2 studies of a universal flu vaccine strategies have also been initiated by NIAID, including an investigational DNA-based vaccine called a DNA prime, followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza vaccines.
There is also one potential universal vaccine that is further down the pipeline. In 2018, BiondVax Pharmaceuticals, based in Israel, launched a pivotal clinical efficacy phase III trial of the M-001 universal flu vaccine candidate, designed to provide multi-season and multi-strain protection against all human influenza virus strains, both seasonal and pandemic. The vaccine contains antigenic peptide sequences shared among many different influenza strains.
“You get nine overlapping peptides that are in an area that is invariant in the virus,” explained Fauci. “If you put them together and vaccinate, we are starting to see a good deal of, if not universal, certainly a broader approach.”
In six completed clinical trials in Israel and Europe, M-001 has been shown to be safe, well-tolerated, and immunogenic to a broad range of influenza strains. An additional Phase II trial in the U.S., sponsored and conducted by NIAID, is ongoing.
Fauci is excited for what is to come.
“We’ve been talking about universal flu vaccines for several years now and we finally have multiple candidates in various clinical trials, including one in a phase III trial,” he said.
Nabel agrees that the future is promising, especially considering how far the field has come in recent years.
“Ten years ago, we would have no understanding of what determinants were being recognized on the virus, where the points of vulnerability would go, what the proofs of concept would be to move it forward and to even have a shot of thinking of universal from a structural point of view,” said Nabel. “I do, however think we have to operate with a sense of urgency; we don’t know when that next major outbreak will come, so the faster we can work, the more we can be informed, particularly about the human immune response and the potential for protection in humans, the quicker we will get to our goal.”
Filed Under: Infectious Disease




