[Image courtesy of Intel]
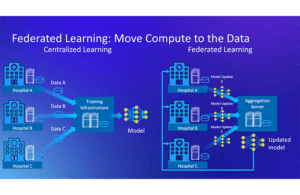
The study made use of federated learning – a distributed machine learning (ML) approach – to facilitate the identification of malignant brain tumors without compromising patient privacy.
The research led to a 33% improved capability to detect brain tumors compared to an AI model trained on public data. Specifically, the federated learning approach led to improved identification and prediction of boundaries in three tumor sub-compartments.
The largest medical federated learning study
The Federated Tumor Segmentation Project was the industry’s largest and most diverse medical federated learning study using federated learning to detect malignant brain tumors.
The data from the research effort were published in Nature Communications.
Accounting for almost half of primary malignant brain tumors, glioblastoma remains one of the most treatment-resistant cancers, according to the National Brain Tumor Society. About 10,000 individuals in the U.S. die from glioblastoma each year. The five-year survival rate for the cancer type is only 6.8%.
While the survival rates for many cancers have improved in recent decades, drug companies have struggled to develop therapies to improve glioblastoma survival rates. Traditional treatments for the cancer include surgery, radiation and chemotherapy.
The advantages of the federated learning approach
The federated learning approach also addressed a number of data science challenges traditionally at work when data are centralized into silos for processing. “That issue can slow the rate of machine learning innovation and the tasks that can be solved using machine learning,” Martin said.
Conversely, the federated learning approach splits up the model training computation and pushes processing to local data silos — rather than a central one. As a result, the approach only sends model updates to a central entity known as an aggregator.
“The aggregator takes and combines all of the partial models into a new global model or a consensus model as we refer to it in the Nature Communications paper,” said Jason Martin, principal engineer at Intel, in a media roundtable event. “And then it sends that back out to each of the data silos for additional training, and this repeats in a cycle called a ’round.’ And in this setting, the data doesn’t need to move from the silo at all.”
Each hospital participated in the project by using compute infrastructure on local data. “And they perform a portion of the training algorithm locally and then send their updates to a centralized aggregation server,” Martin said. In the federated tumor segmentation project, the University of Pennsylvania hosted the aggregation server.

[Image courtesy of Intel]
Looking to the future
An improved understanding of glioblastoma could results in better outcomes over the long term.
“We’re looking forward to ways to set up federations that are persistent and make it easier for additional use cases to experiment on them,” Martin said.
Intel foresees applying the federated learning project’s experience to various medical research types.
“I also think there are some wonderful opportunities to do sort of meta-studies across federations in terms of fairness,” Martin said.
Intel plans on prioritizing data security in future projects that rely on federated learning. “As you see data residing in its natural distribution across institutions, you can start to identify some of the other trustworthy and ethical AI issues across those institutions,” Martin said.
Filed Under: Data science, Oncology, Special Feature




Tell Us What You Think!
You must be logged in to post a comment.