BriaCell Therapeutics is on the path to a cancer vaccine. Its investigational therapy, BriaVax, is a genetically engineered whole-cell vaccine intended to treat advanced breast cancer patients. Derived from a human breast cancer cell line, it is believed to activate the immune system to recognize and eliminate cancerous cells by inducing tumor-directed T cell and potentially antibody responses.
The immune-oncology focused biotechnology company has demonstrated encouraging clinical results, and is intent on building upon these findings to further advance BriaVax through additional FDA-approved clinical trials to help cancer patients with limited therapeutic options.
We found out more about the vaccine’s potential and their pipeline in an exclusive interview with William V. Williams, MD, president and CEO, BriaCell Therapeutics Corp.
Drug Discovery & Development: What is BriaVax?
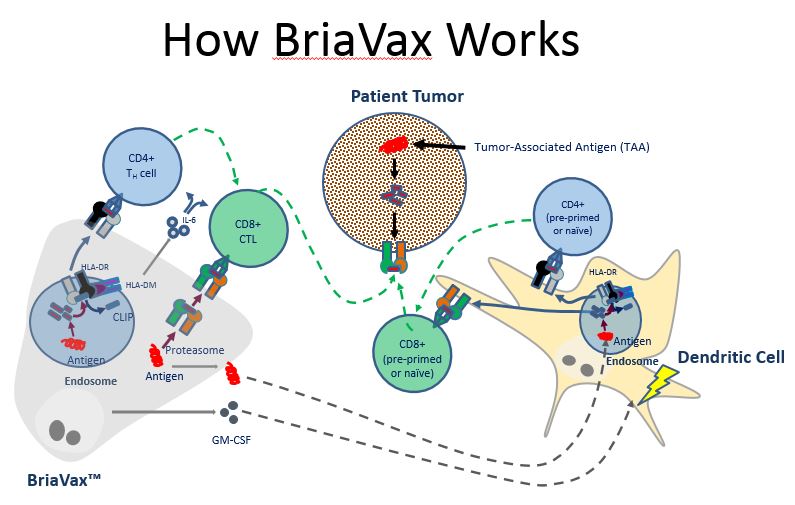
William V. Williams: BriaVax is a Her2/neu positive breast cancer cell line that BriaCell engineered. Her2/neu is a protein well known for its overexpression in breast cancer but also associated other epithelial malignancies including ovarian, pancreatic, colon, bladder and prostate cancers. It produces and secretes granulocyte-macrophage colony-stimulating factor (GM-CSF), a protein that promotes the immune response by activating dendritic cells, which are potent antigen-presenting cells.
BriaVax is unique in that it expresses both class I and class II Human Leukocyte Antigen (HLA) molecules, which are known to stimulate CD8+ cytotoxic T cells and CD4+ helper T cells responses. We therefore hypothesize that particularly strong tumor-directed immune responses occur if BriaVax directly activates CD4+ and CD8+ T cells that recognize BriaVax antigen/HLA combinations which are also expressed on the patient’s cancer cells.
DDD: How was it developed?
WW: Treatment with BriaVax is the result of decades of clinical investigation and research on therapeutic cancer vaccines, especially in the laboratory of Dr. Charles Wiseman, including a human breast tumor cell line (SV-BR-1), which has been genetically engineered to secrete GM-CSF and is now referred to as BriaVax. GM-CSF has multiple effects on the tumor-immune response equilibrium, including dendritic cell recruitment, activation, and maturation.
For clinical use, BriaVax cells are irradiated to prevent cell replication. Two days before BriaVax is injected into four intradermal sites, the patients are pre-treated with low-dose cyclophosphamide to take the “foot off the brake” with respect to regulatory T cell response. Two and four days after the injections, interferon-alpha (IFN-alpha) is injected into the same sites to “step on the gas” and evoke a prolonged immune response.
DDD: How does it work against late stage breast cancer?
WW: We believe BriaVax awakens the patient’s immune system to recognize tumor cells as foreign, and hence destroys them. We hypothesize BriaVax exerts its mechanism of action by several means:
- BriaVax contains breast cancer antigens which can be taken up by antigen-presenting cells (APCs), such as dendritic cells, to stimulate anti-cancer CD4+ T helper cells and CD8+ cytotoxic T cells. The GM-CSF secreted by BriaVax further augments this immune response by enhancing the functionality of the APCs.
- BriaVax cells may also directly transfer HLA molecules loaded with breast cancer antigens to the APCs by a process called trogocytosis. Such APCs would then further stimulate the immune response by activating anti-cancer T cells.
- BriaVax cells might function directly as APCs for previously activated (i.e., not naïve) T cells, thereby further stimulating the anti-cancer immune response.
At least this last feature, acting directly as APCs, appears unique for BriaVax and may account for its potent anti-cancer activity in some cases. By undertaking a gene expression analysis we identified several genes encoding tumor-associated antigens (TAAs) that are overexpressed in BriaVax cells and might be relevant for all of the aspects outlined above of BriaVax’s hypothesized mechanism of action. BriaVax also appears to express several other potent immune stimulatory molecules, such as IL-6 and IL-15, which might further activate the immune system.
DDD: What clinical data do you have?
WW: In a clinical study, we treated four evaluable patients with advanced cancer. One of them had a remarkable response, with metastatic breast cancer shrinking markedly in several locations. The protocol at that time only permitted five months of treatment, and when the treatment was stopped the breast cancer came back. The patient was treated again and her cancer, including brain metastases, responded again to BriaVax. Interestingly, this patient matched BriaVax at 2 HLA loci. HLA (Human Leukocyte Antigen) molecules are the molecules that bind cancer antigens and “present” them to anti-cancer T cells. This double match may both help explain how the treatment works and provide a screening test to allow us to select the patients most likely to respond to BriaVax.
We also observed a linear relationship between BriaVax exposure and the rising levels of soluble CD40 Ligand (sCD40L) in a subject with a strong anti-tumor effect. The membrane-bound form, CD40L, is a protein that is expressed on the cell surface of components of the immune system. CD40L is known as one of the strongest stimulants of the immune system resulting in dendritic cell maturation, and rising levels of CD4+, CD8+, and NK cells, i.e., immune cells known for their anti-tumor activities. Additionally, the CD40/CD40L pathway is critical for the development of protective anti-tumor activity by providing a key initial step in the development of humoral (B cell/antibody-mediated) immunity. sCD40L levels may provide a predictor of response to BriaVax. Our preliminary analyses have also shown several up-regulated genes in BriaVax that encode proteins known to be immunogenic (i.e., immune response-generating) and perhaps enhance immune responses in patients who receive BriaVax.
DDD: Where are you in the development process?
WW: We are currently in the initial Phase I/IIa study which is designed to recruit 25 – 40 subjects using monotherapy with BriaVax.
A roll-over protocol was recently approved by FDA which will permit combination therapy with a checkpoint inhibitor if patients progress on monotherapy. We should also have data on our first 10 patients by the first quarter of 2018. Our goal is to gather enough data to progress to an End of Phase 2 (EOP2) meeting with the FDA in 2019 and begin a pivotal registration study that year.
And, if all goes well, we anticipate filing a Biologics License Application (BLA) with the FDA in 2022. Perhaps we can go to market the same year.
DDD: What are your future plans for the company?
WW: We are developing second generation BriaVax cell lines, which are planned to express several immunostimulatory genes, as well as versions with different HLA molecules. If our hypothesis is correct, we may need to match the HLA type of each patient to a particular version of BriaVax to obtain the most robust response. With second generation BriaVax, we should be able to match the vaccine to the patient, and provide benefit to many more patients.
We also have a robust pipeline, including small molecule inhibitors of protein kinase C delta (PKCδ).We know that RAS transformation is seen in ~30 percent of all cancers.RAS depends on PKCδ activity to avoid being degraded.When PKCδ activity is inhibited, RAS is degraded and RAS transformed cancers undergo apoptosis, i.e., programmed cell death.Also, transforming growth factor beta (TGF-β) signals through PKCδ, and TGF-β is an immunosuppressive molecule, turning off the immune response.So PKCδ inhibitors will likely have immunotherapy activity as well.We already have data that potent and selective PKCδ inhibitors have activity against a number of cancers, including pancreatic cancer, melanoma, neuroendocrine tumors (such as carcinoid), and breast cancer.We are developing these PKCδ inhibitors with the goal of entering the clinic by 2019.
Filed Under: Drug Discovery




