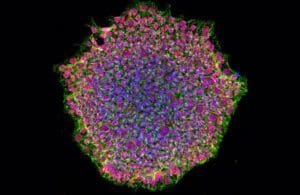
Human induced pluripotent stem cell colony. [Image courtesy of NIH]
CAR-T therapies are becoming the standard of care for some relapsed or refractory diseases, such as multiple myeloma, acute lymphoblastic leukemia, follicular lymphoma, and some forms of aggressive, non-Hodgkin lymphoma. In studies with autologous cell therapies (tisagenlecleucel and axicabtagene ciloleucel), complete response rates (CRR) ranged from 40-57%, while a mix of salvage therapies yielded only a 7% CRR (1). Despite the treatment advantage, manufacturing CAR-T is limited by the availability of the patient’s cells, which can be affected by the progression of disease and the need for up to three weeks of processing time (leukapheresis to treatment) for the autologous cells to be engineered, expanded, and made available for administration (2). Besides cell source and shipping logistics, available manufacturing capacity, quality control, high cost of goods and processing failures are genuine challenges (3). Inadequate cell expansion for autologous CAR-T can lead to process failure rates as high as 14% (4), although this may improve as manufacturers gain experience. In addition, autologous cells may be released to the clinic even if the final product does not meet specifications since there may be no alternative for the patient. In five clinical trials, 6-10% of the autologous CAR-T cell products were out of specification but still released for clinical use (5).
Allogeneic cells can provide an available therapy without the wait for the manufacturing of therapeutic cells or reliance upon the patient’s cells that may have been compromised by previous treatments or health state. However, an allogeneic approach has challenges. There are not always readily available cell sources, and complexities inherent to genetically engineering specificity to enable tumor targeting, avoiding host rejection of allogeneic cells, and maintaining cell function all pose potential problems. Overcoming these requires an allogeneic, off-the-shelf source of therapeutic cells. This can translate to shorter time to treatment, removed variability of patient-sourced cells, readily available, consistent supplies, and increased overall accessibility to critically needed cell therapies. In addition, since the treatment does not have to be linked to a specific patient like autologous treatments, the supply chain can be flexible and simplified.
Advantages of allogeneic, iPSC-based cell therapeutics
Although allogeneic approaches provide a more reliable cell source than individual autologous donors, donor cells must be screened and tested. This can be challenging for some allogeneic processes that need to repeatedly source from multiple donors and repeat the cell engineering to produce different batches. Engineering is, therefore, part of the manufacturing process. Since gene editing methods are not 100% efficient, a homogenous product cannot be produced since only some cells carry all intended edits. Gene editing may also not always accurately modify the desired location, which increases the risk of off-target effects that cannot be characterized in a heterogenous cell bank.
In contrast, iPSCs rely on a single donor and reprogramming operation. Therefore, a single donor can be used for the lifetime of a given product. Hence, a reliable parental iPSC bank can be established and well-characterized (Figure 1).
Precision genomic engineering, using CRISPR-mediated homologous directed repair (HDR), can be performed at the iPSC stage to impart desired characteristics such as tumor targeting, immune evasion (6), and cell persistence. Editing can be conducted sequentially, and cells with the intended edits and no unwanted off-target edits can be selected for each subsequent step, ensuring every cell contains all desired edits and the batch is homogenous. Next, a clone from this engineered iPSC can be isolated and expanded into master and working cell banks. This enables the examination of functional, phenotypic, and genomic characteristics before cells are expanded and differentiated into effector cells. In this way, a consistent cell therapeutic with well-defined attributes can be repeatedly manufactured.
Process challenges
Once the engineered iPSC has been designed, the process will have the ability to generate many large-scale lots from a single bank and production can be expanded at a commercial scale to meet demand, bring economies of scale, and enable lower cost of goods and greater availability of supplies.
With iPSCs, advances in bioreactor technology can be leveraged to increase scale using various equipment types. The use of larger scales enables the use of several systems to produce cells, from small- to large-scale bioreactors, as well as static and dynamic systems (e.g., G-Rex devices, Xuri bags, PBS Biotech vertical wheel bioreactors, Biostat stirred tanks). Selection depends on the desired scale and cell performance and differs on the stage of development and expected commercial scale. However, leveraging existing biopharmaceutical cellular processes (used for proteins and vaccines) can only be applied to a limited extent since they have been developed to grow cells for secreted/expressed therapeutics rather than for cells themselves (except cell banking). As a result, cell therapeutics challenges include maintaining integrity, health and activity through the expansion, differentiation, formulation, and delivery processes.
iPSC sourcing advantages
Allogeneic processes can differ in cell type and number of donors. While some require multiple donors due to the limited expandability of primary cells ex vivo, iPSCs have a self-renewing capacity that allows expansion beyond the amounts needed for the lifetime of a commercial product. In addition, a single-sourced iPSC bank allows consistent starting material that can be characterized. Since there is only a single donor, donor variability is not a consideration for iPSC-based, allogeneic cell therapy developers such as Century Therapeutics, which can leverage an expandable, consistent supply as starting material to engineer and produce iPSC-derived T (iT) and NK (iNK) therapeutic cells.
Defined cell engineering
Another advantage of an iPSC-based approach, compared to autologous and multiple donor allogeneic sources, is that clonal, gene-edited iPSC can be generated and banked to form the basis of multiple progenitor and terminally differentiated cells. In this way, the genetic editing sequence is performed once and is the source of all subsequent expansion and differentiation operations. This ensures consistency since genetic modifications are not repeated for different lots, which can lead to potential variability in modifications. In addition, the edited iPSC can be clonally isolated, selected, and fully characterized to ensure consistency and performance. The clonal cells can then be expanded into master and working cell banks. Creating and utilizing cell banks also allows for thorough characterization and control. As a result, the number of population-doubling levels (PDL) is more consistent, and the characterization of the genetic modifications is well-defined, along with a more reliable cytotoxicity assessment. The challenge then becomes the development of assays that monitor the potency-indicating functions of the cell, such as target cell killing, persistence, autocrine functions, and related characteristics.
Facility considerations
The use of a centralized facility is preferred for allogeneic cell therapy. However, distributed facilities would not be advantageous since the product can be readily available either immediately if in stock or shipped on demand from storage. This has the additional advantage of increased quality control over large batches.
Designing a flexible facility allows the process to be selected for the application and cell type. Both T and NK cells can have different methods due to unique requirements for differentiation and the number of cells required to meet dosage needs. A good facility design will accommodate differences in equipment type and scale.
The ability to make relatively large lots will also allow us to leverage bioreactor and process monitoring innovations and state-of-the-art banking characterization. This is expected to allow efficiencies as more experienced is gained to ensure consistent and highly functional cells.
Figure 1. Manufacturing processes for autologous and allogeneic cell therapies. Graphics were created in BioRender.
Cryopreservation and distribution to patients
Once scale-up of the process is achieved, cryopreservation of an iNK or iT product is required for broad distribution, stable storage at a local site, and patient availability. Optimization of cryopreservation involves the development of a suitable preservative formulation and a freezing, distribution, and thawing process to ensure minimal loss of potency. NK cells are more challenging than T cells since they are sensitive to cryopreservation.
Additional considerations
The allogeneic approach must address the major shortcomings of autologous cell therapies, namely limited manufacturing capacity that restricts availability, the speed at which therapies can be made available, and the cost of manufacturing. Each of these factors have been addressed in traditional biopharmaceutical processes and has their parallels here. However, the potential of producing thousands of vials at a time and having them available at a site almost immediately is attainable.
Addressing the cost of goods is feasible but also presents a significant challenge. Although larger manufacturing scales typically result in a lower cost of goods per batch based on volume discounts of ancillary materials, cell processes involve the use of cytokines and other factors that are typically limited in capacity and number of vendors. The price of these components can be expected to decrease as vendors increase efficiency and productivity. However, the number of compounds needed and the limited availability of vendors will necessitate more attention to achieve this goal.
Conclusion
The use of iPSC-derived cell therapies has the potential to allow more readily available, consistent product at larger scales, however, work remains to ensure the quality and cost of critical components are controlled. If we can achieve this, we can maximize the accessibility of iPSC-based therapeutics and consistently produce safer products for patients.
References
- Institute for Clinical and Economic Review, Final Evidence Report – CAR-T Therapies for B-Cell Cancers (2018), https://icer.org/assessment/leukemia-and-lymphoma-2018.
- Kansagra A, Farnia S, Majhail N, Expanding Access to Chimeric Antigen Receptor T-Cell Therapies: Challenges and Opportunities, ASCO Educational Book 2020: 40, e27-e34.
- Burke CJ, Zylberberg C, Sources of Variability in Manufacturing of Cell Therapeutics. Regen Eng Transl Med 5, 332–340 (2019). https://doi.org/10.1007/s40883-019-00130-5.
- Bersenev A, CAR‐T cell manufacturing: time to put it in gear, 2017 Transfusion 57(5):1104-1106, DOI: 10.1111/trf.14110
- Bersenev A, Kili S, Management of ‘out of specification’ commercial autologous CAR-T cell products, Cell Gene Therapy Insights 2018; 4(11), 1051-1058. DOI: 10.18609/cgti.2018.105.
- Borges L, Levitsky H, The Challenge Of Immune Rejection For Allogeneic Cell Therapies, https://www.bioprocessonline.com/docpreview/the-challenge-of-immune-rejection-for-allogeneic-cell-therapies-0001/6935c492-f2ef-452a-aff1-5298036c819c.
 Carl Burke is the vice president of CMC operations and external technologies at Century Therapeutics. He joined Century Therapeutics in 2019 and established the CMC and quality functions. Before joining Century, Carl was involved in the development and manufacturing of both allogeneic and autologous cell therapies and their regulatory filings at Janssen. His earlier responsibilities were leading drug product development area for biologics and subsequently leading CMC development and pilot plants in Janssen Vaccines. Carl’s prior work at Merck involved vaccine and biologics product development for all phases of development and commercial support.
Carl Burke is the vice president of CMC operations and external technologies at Century Therapeutics. He joined Century Therapeutics in 2019 and established the CMC and quality functions. Before joining Century, Carl was involved in the development and manufacturing of both allogeneic and autologous cell therapies and their regulatory filings at Janssen. His earlier responsibilities were leading drug product development area for biologics and subsequently leading CMC development and pilot plants in Janssen Vaccines. Carl’s prior work at Merck involved vaccine and biologics product development for all phases of development and commercial support.
Filed Under: Cell & gene therapy




Tell Us What You Think!
You must be logged in to post a comment.