 Cubist Pharmaceuticals Inc. announced that the European Medicines Agency (EMA) has accepted for review the company’s Marketing Authorization Application (MAA) for its investigational antibiotic ceftolozane/tazobactam. Cubist is seeking approval of ceftolozane/tazobactam for the treatment of complicated urinary tract Infections and complicated intra-abdominal infections, with a decision from the European Commission (EC) expected during the second half of 2015.
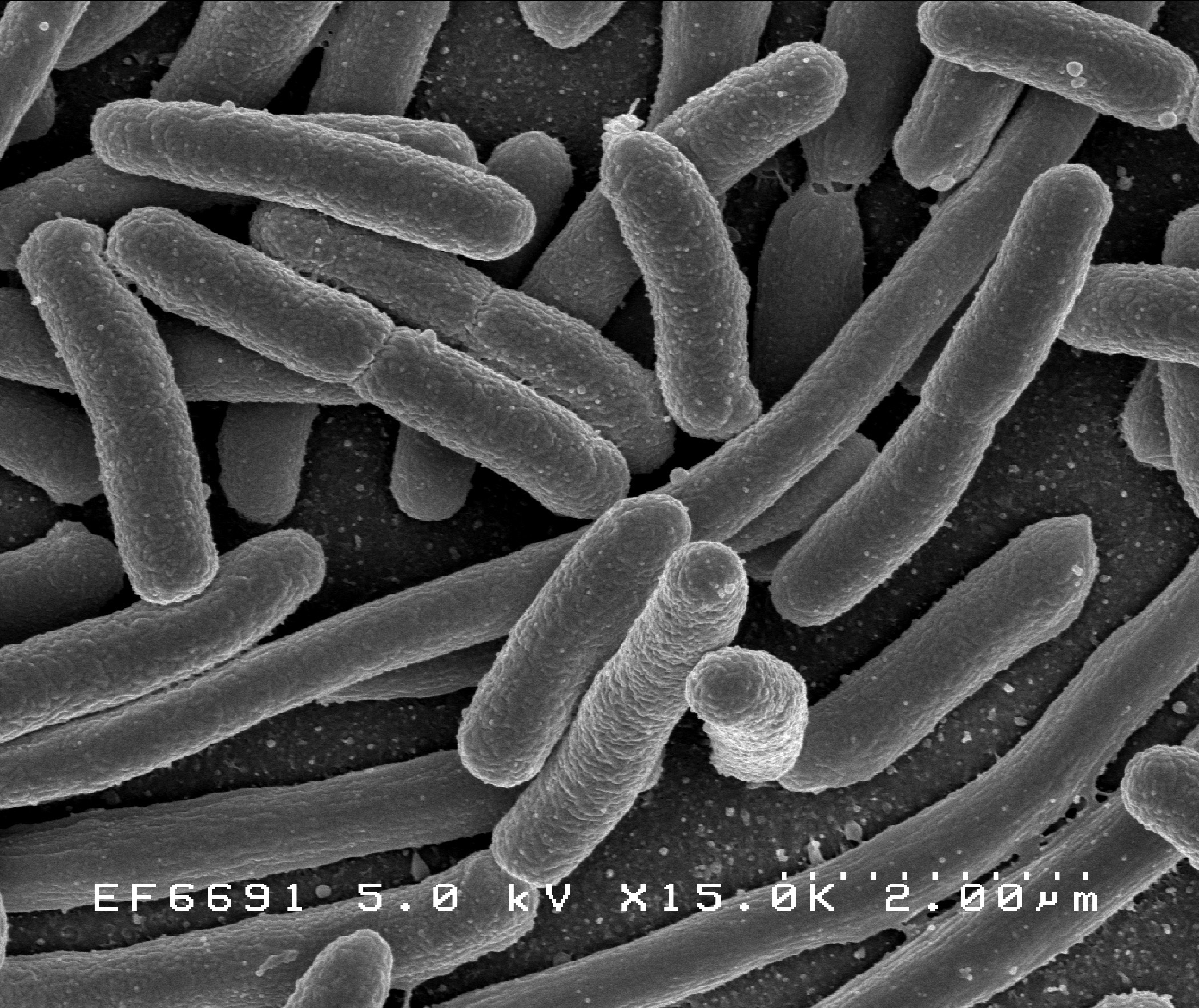
Cubist Pharmaceuticals Inc. announced that the European Medicines Agency (EMA) has accepted for review the company’s Marketing Authorization Application (MAA) for its investigational antibiotic ceftolozane/tazobactam. Cubist is seeking approval of ceftolozane/tazobactam for the treatment of complicated urinary tract Infections and complicated intra-abdominal infections, with a decision from the European Commission (EC) expected during the second half of 2015. The MAA submission is based on positive data from two pivotal Phase 3 clinical trials of ceftolozane/tazobactam in complicated urinary tract infections and complicated intra-abdominal infections. These studies met both the EMA and U.S. Food and Drug Administration (FDA) specified primary endpoints. Results of the secondary analyses were consistent with and supportive of the primary outcomes. In the clinical trials, ceftolozane/tazobactam demonstrated activity against problematic Gram-negative bacteria, including Pseudomonas aeruginosa and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli (E. coli) and Klebsiella pneumoniae in patients with complicated infections.
“We are pleased to receive MAA acceptance for ceftolozane/tazobactam and look forward to working with the EMA on this important review,” said Steven Gilman, executive vice president of Research and Development and Chief Scientific Officer of Cubist. “As we continue to expand globally, this advancement further positions Cubist to respond to growing health threats and reinforces our commitment to bring new antibiotics to patients worldwide facing serious infections, including those caused by Gram-negative bacteria.”
Prior to the EMA acceptance of the MAA, the FDA accepted the company’s New Drug Application (NDA) for ceftolozane/tazobactam with Priority Review and assigned an action date of December 21, 2014.
Date: August 22, 2014
Source: Cubist Pharmaceuticals
Filed Under: Drug Discovery




