Flow chemistry techniques are increasingly being used in drug discovery to provide cost-effective access to a wide range of structurally diverse small molecule analogs, as well as access to previously underused or inaccessible chemistries. There are several ways that this powerful technique can be used to increase structural diversity when building candidate molecules, including linear…
Genentech’s lab in the loop aims to tap the power of quantity for quality drug discovery
We can design chips that power self-driving cars and create physically-realistic video footage based on text descriptions. Yet, as Genentech’s Aviv Regev pointed out in a session about the company’s lab in the loop at NVIDIA’s GTC conference, the humble cells within us operate with a complexity that still eludes our full understanding. It turns out that a cell is itself like a computational device…
NVIDIA exec on how ‘NIMs’ can help biopharma navigate the challenges of deploying generative AI
The buzz surrounding generative AI may be undeniable, but its real-world impact on heavily-regulated sectors like drug discovery continues to evolve. Consequently, most drug candidates, circa 90%, continue to fail. Kimberly Powell, vice president of Healthcare at NVIDIA, believes that a new microservices-based offering known as NIMs (NVIDIA Inference Microservices) could help pharma firms navigate…
NVIDIA expands BioNeMo platform with new foundation models and microservices for AI-powered Drug Discovery
NVIDIA is putting the power of generative AI for drug discovery into the hands of more pharmaceutical and biotech companies with an expanded collection of AI models and flexible deployment options. More than 100 firms are already using the company’s biomolecular BioNeMo platform to accelerate the development of therapeutics. “For the first time in history,…
Denmark teams up with Novo Nordisk Foundation, NVIDIA to launch visionary AI research center
A collaboration between the Novo Nordisk Foundation, the Export and Investment Fund of Denmark (EIFO), and NVIDIA will establish a national AI Innovation Centre in Denmark focused on accelerating research and innovation in fields including healthcare, life science, and quantum computing. The initiative is led on the Danish side by the Novo Nordisk Foundation, which…
Iambic Therapeutics and NVIDIA partner to slash cancer drug development timelines
Using generative AI in drug discovery, Iambic Therapeutics (formerly Entos) has advanced its IAM1363 drug candidate from program launch to clinical studies in fewer than 24 months — a process that often takes several years. Iambic Therapeutics’ AI drug development milestone relied on an alliance with NVIDIA researchers and engineers and through the use of…
Q&A: How Insilico Medicine’s AI identified a new IPF drug target in record time
Idiopathic Pulmonary Fibrosis (IPF), a devastating lung disease affecting millions with increasing incidence, may have a new treatment hope thanks to a novel inhibitor of TNIK, a kinase newly implicated in fibrosis, identified using generative AI drug discovery platforms in just 18 months. Researchers at Insilico Medicine, along with international collaborators, harnessed the power of…
Could Wegovy’s cardiovascular label expansion be a catalyst for GLP-1 obesity drug coverage?
The recent FDA approval of a cardiovascular risk reduction indication for Wegovy (semaglutide) could point toward a significant opportunity for pharma companies seeking to reshape payer perceptions and expand coverage for next-gen metabolic therapies. This regulatory shift, allowing Wegovy to be prescribed for reducing the risk of major adverse cardiovascular events such as heart attack…
Lantern Pharma aims to take drug to phase 3 for $100-200 million with AI-powered approach
Lantern Pharma’s AI-powered sprint Lantern Pharma (NASDAQ: LTRN), a publicly traded clinical-stage biotech company with a market cap of around $79 million as of mid-March 2024, is shooting for developing $200 million drugs with a machine learning-based platform. The oncology-focused firm Lantern Pharma, profiled last year, has advanced its new drug (LP-284) to a Phase…
In 2023, Roche and Novartis led the pack in drug pipeline scale
When reviewing R&D spending trends for 2023, Merck & Co. is a clear outlier given its decision to count its $10.3 billion Prometheus acquisition as an R&D charge. In all, the company committed more than half of its revenue to R&D. But Swiss giants Roche and Novartis remain frontrunners in terms of their pipeline of…
Navigating the cancer progression pathway with liquid biopsy
Tumor heterogeneity analysis is a challenging but critical step for the advancement of cancer therapy research. DNA mutation interrogation and gene expression pattern evaluation are crucial for predicting a tumor’s response or resistance to specific drug or hormone treatments. Liquid biopsy has emerged as a promising tool, offering a rapid, minimally invasive method for early…
FDA fast-tracks psilocybin-based CYB003 for depression
The FDA continues to signal openness to psychedelic-based therapies. Following its recent Breakthrough Therapy Designation (BTD) for a potential LSD-based anxiety treatment, the agency has extended the same status to Cybin Inc.‘s CYB003 for major depressive disorder (MDD). The move marks the first FDA Breakthrough Therapy Designation for an adjunctive, psychedelic-based Major Depressive Disorder treatment.…
How Novo Nordisk’s Wegovy cardiovascular benefits could drive further growth
Semaglutide was already one of the best-selling drugs of recent memory. And Novo Nordisk the fastest-growing Big Pharma firm. Now, the FDA’s decision to expand the label of its weight-loss version of the drug to include cardiovascular benefits could help unlock more growth momentum for Novo Nordisk. This positions Wegovy as the first weight-loss medication…
Novo Nordisk achieves 74% growth surge, clinching fastest-growing pharma title
No longer just a diabetes company, Novo Nordisk’s semaglutide-based therapies have fueled its rise to pharma powerhouse status. From 2021 to 2023, the company cemented its position as the fastest-growing Big Pharma player, reaching a 35% year-over-year growth rate in 2023 and 74% growth from 2020 to 2023 when measured in U.S. dollars. While Pfizer…
Top pharma companies ranked by 2023 R&D spend
Which major pharmaceutical company leads the way in research and development? Data reveals that the top R&D spender in 2023 was Merck & Co., by a wide margin. While Pfizer had almost as much revenue as Merck ($60.1 billion vs. $58.5 billion), its R&D ratio was on the lower end at 18.29%, or $10.7 billion.…
Why Merck shelled out $30B+ on R&D in 2023
In 2023, Merck & Co. made a considerable investment in research and development, spending more than $30 billion. This figure represents more than double the company’s R&D spending ($13.5 billion) in 2022. The closest competitor in terms of R&D investment was Roche Pharmaceuticals, with a 2023 R&D expenditure of roughly $14.7 billion. This sum is…
Could LSD change the game in anxiety treatment?
A once-controversial psychedelic substance could potentially be a promising treatment for generalized anxiety disorder (GAD). That’s the view of Dr. Rakesh Jain, a psychiatrist with extensive experience in clinical practice, research, and education, affiliated with Texas Tech University School of Medicine. Jain expressed optimism in LSD-based therapy while acknowledging the challenges inherent in such a…
FDA grants breakthrough status to LSD-based anxiety treatment
In a major shift away from decades of stigma, the FDA has granted Breakthrough Therapy Designation to MindMed‘s MM120, an LSD-based treatment for Generalized Anxiety Disorder (GAD). This milestone not only underscores the growing recognition of the therapeutic potential of psychedelics but also could point to a potential turning point for the struggling psychedelic sector…
Are Big Pharma giants getting the right ROI on their R&D investments? A visual exploration
While annual reports show broadly similar R&D strategies among Merck & Co., Pfizer, Johnson & Johnson and AbbVie, their 2020–2023 financial metrics reveal a concerning trend. Merck & Co. may be the new top dog of Big Pharma, but the firm’s 1.4% revenue growth in 2023 represents a significant slowdown. Pfizer’s 41.7% revenue decline from…
Debiopharm’s multilink technology and partnerships drive oncology pipeline strategy
Debiopharm, an independent Swiss biopharmaceutical company based in Lausanne, seeks to carve a niche in the competitive oncology and infectious disease markets. Its business model focuses on in-licensing promising drug candidates from universities and smaller biotechs, aiming to add value through development. (The company is also partnering with AI-focused firms like VeriSIM Life.) Sandra von…
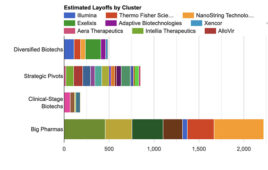
Biotech layoffs in 2024: Identifying common threads among affected companies
Companies across the industry have contributed to biotech layoffs in early 2024, driven by factors ranging from operational restructuring to strategic pivots and clinical trial failures, with major hubs like California and Massachusetts bearing the brunt of the impact. A recent analysis assembled with the help a machine learning technique known as clustering reveals a pattern of job…
Anosmia to amyloidosis: nference’s AI is decoding healthcare data at scale
The complexity of patient records, with their mix of unstructured notes and diverse data types, defies traditional analysis. While a thorough review of even a single patient’s file can be tedious for a human, AI tools offer the power to analyze tens or even hundreds of millions of records, unlocking data patterns that would otherwise…
Exploring the forces behind 2024 biotech layoffs: A visual journey
Biotech layoffs continue to pummel the industry in early 2024 — albeit at a slightly lower clip than in 2023. Smaller firms continue to bear the brunt of funding woes and disappointing clinical data. From January to February, operational reorganizations fueled 45 layoff announcements, followed closely by strategic pivots in 37 events. Other common drivers…
Where are the top Big Pharma hubs in 2023?
By analyzing the financials of dozens of Big Pharma firms, we can pinpoint the global hubs that drive much of the industry’s innovation. This analysis reveals regions like New York, New Jersey, Tokyo, Basel, and Frankfurt metro area as core centers for pharma revenue and R&D spending. Unsurprisingly, the area with the densest concentration of…
Moving the needle on diversity in clinical trials: Where do we go from here?
Enhancing patient diversity in clinical trials has become a key priority in drug development. The main concern is that critical data that includes underrepresented patient populations is being left out as many clinical trials do not reflect all populations that may eventually take a therapy. These underrepresented groups consist of women, including those who are…