 A drug candidate that overstimulates proteins crucial for tumor growth shows promise as a new strategy to treat a wide range of cancers. The demands of rapid cell division put a strain on cancer cells, and the approach works by tipping cell stress over the edge. In the August 10 issue of Cancer Cell, American researchers show that the drug candidate inhibits tumor growth in a mouse model of breast cancer and efficiently kills a broad range of human cancer cells.
A drug candidate that overstimulates proteins crucial for tumor growth shows promise as a new strategy to treat a wide range of cancers. The demands of rapid cell division put a strain on cancer cells, and the approach works by tipping cell stress over the edge. In the August 10 issue of Cancer Cell, American researchers show that the drug candidate inhibits tumor growth in a mouse model of breast cancer and efficiently kills a broad range of human cancer cells.“No prior drug has been previously developed or proposed that actually stimulates an oncogene to promote therapy,” says co-senior study author David Lonard of Baylor College of Medicine. “Our prototype drug works in multiple types of cancers and encourages us that this could be a more general addition to the cancer drug arsenal.”
Because cancer cells acquire mutations in oncogenes—genes that can transform cells into cancer cells—to support their growth and survival, a great deal of research has focused on identifying oncogenes that could be targeted by cancer drugs. Members of the steroid receptor coactivator (SRC) family of oncogenes are especially promising as therapeutic targets because these proteins sit at the nexus of key signaling pathways that cancer cells use to quickly grow, spread to other tissues, and acquire drug resistance. In a previous study, Lonard and co-senior study author Bert O’Malley of Baylor College of Medicine screened a large number of compounds to identify SRC-inhibiting molecules that kill a wide variety of cancer cells and inhibit tumor growth in animal models.
These compounds are similar to conventional therapies designed to inhibit the activity of key cancer oncogenes. But Lonard and O’Malley had a counterintuitive idea: what if they could disrupt key signaling pathways and kill cancer cells by overstimulating SRCs? After all, cancer cells rely heavily on SRCs to delicately orchestrate a wide range of cellular events, so SRC stimulation might be just as effective as SRC inhibition at disrupting the balance of signaling activity in cancer cells.
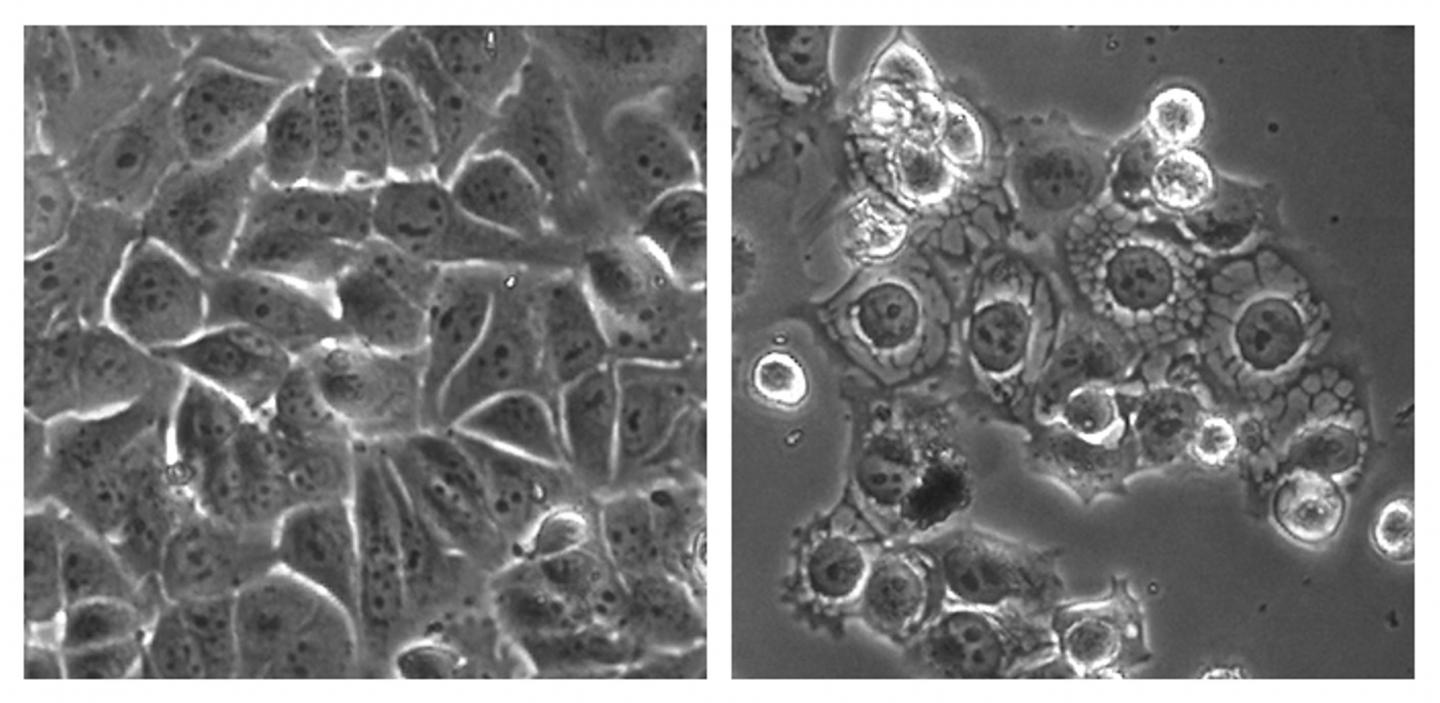
To test this idea, they screened hundreds of thousands of compounds to identify a potent SRC activator called MCB-613. This compound killed human breast, prostate, lung, and liver cancer cells, while sparing normal cells. When the researchers administered MCB-613 to 13 mice with breast cancer, the drug candidate almost completely eliminated tumor growth without causing toxicity, whereas tumors continued to grow by about 3-fold over 7 weeks in the control group of 14 mice.
MCB-613 killed cancer cells by causing the accumulation of unfolded proteins in a cell structure called the endoplasmic reticulum (ER). To support their rapid proliferation, cancer cells must synthesize a large number of proteins, putting a strain on the ER to keep up with its heavy workload of properly folding proteins. Overstimulation of SRCs places extra demands on the ER when it is already operating at maximum capacity, causing the accumulation of a large number of unfolded proteins. This triggers a cell stress response that in turn causes the build-up of toxic molecules called reactive oxygen species.
Taken together, the findings suggest that elevating SRC activity beyond the already high levels present in cancer cells further pressures their maximized stress response system and selectively kills them. In future studies, the researchers will continue to explore the mechanisms by which SRCs kill cancer cells and will screen for even better SRC activators. “We are optimistic that these drugs will eventually find their way into the clinic for use in patients,” O’Malley says.
Source: Celll Press
Filed Under: Genomics/Proteomics




