 Gone are the days when Microsoft’s animated paperclip, Clippy, popped up on your screen with helpful — and sometimes annoying — tips and tricks for your Word document. Now, the Israeli eQMS compliance provider Dot Compliance has launched what it hails as an industry-first electronic Quality Management System (eQMS) for life sciences with a prominent generative AI-enhanced digital assistant that could take intelligent assistance to new heights. “It’s not just ChatGPT. It’s many other algorithms that are evolving,” said Doron Sitbon, founder and CEO of the company.
Gone are the days when Microsoft’s animated paperclip, Clippy, popped up on your screen with helpful — and sometimes annoying — tips and tricks for your Word document. Now, the Israeli eQMS compliance provider Dot Compliance has launched what it hails as an industry-first electronic Quality Management System (eQMS) for life sciences with a prominent generative AI-enhanced digital assistant that could take intelligent assistance to new heights. “It’s not just ChatGPT. It’s many other algorithms that are evolving,” said Doron Sitbon, founder and CEO of the company.
Transforming quality assurance with AI-driven quality management software
Rather than Clippy, Sitbon uses another metaphor to illustrate the company’s ChatGPT-enhanced “Dottie” capabilities for its QMS Xpress quality management software. “I think of it as an Iron Man suit for a [quality assurance] manager,” Sitbon quipped. The Dottie digital assistant pops up offering context-based advice on how to deal with specific quality deviations.
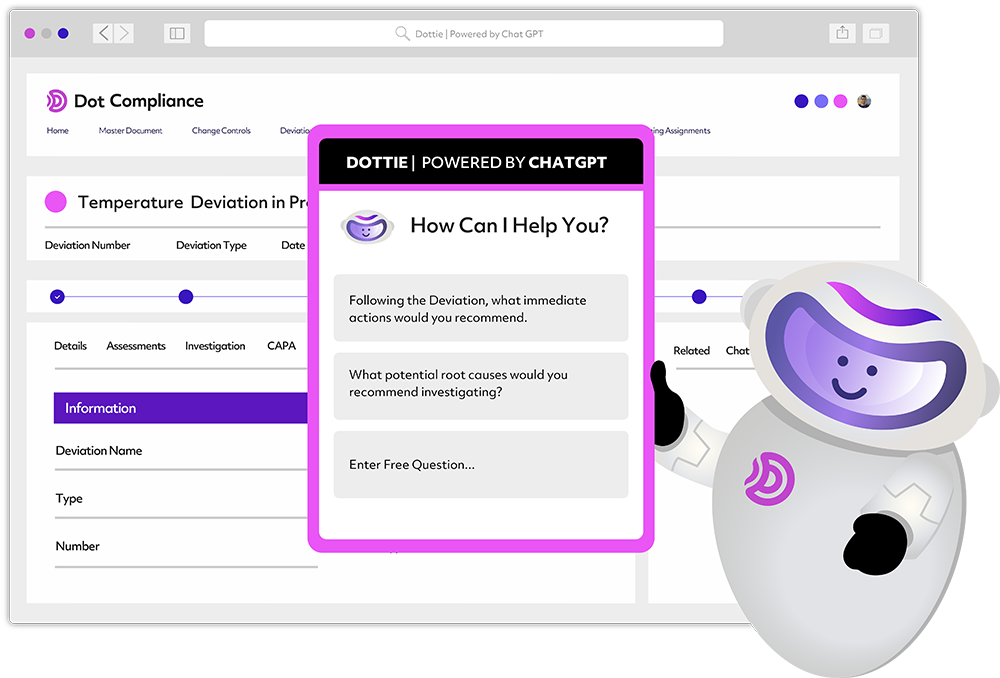
[Image courtesy of Dot Compliance]
Sitbon emphasized that the goal is not to replace the judgment of the QA manager. Instead, the AI-enhanced eQMS allows them to see more, move faster, and parse larger sets of information. “The future is now,” he added.
Dot Compliance believes their quality management software offers QA managers in the life sciences an unparalleled level of support, efficiency, and expertise. “AI would assist the QA manager of the future in analyzing distinct data points into a cohesive picture,” Sitbon said.
For Dot Compliance, the AI itself is not the point. Conversely, the main focus is the combination of generative AI with domain expertise, Sitbon said. Those capabilities could make it possible for non-technical individuals to influence quality assurance workflows more. “Our goal as a vendor is to remove the obstacles, making [eQMS capabilities] available to people that are not technical,” Sitbon said. “You have to take the domain knowledge and technological expertise and create a blend that makes sense to users.”
Overcoming regulatory hurdles and boosting efficiency in life sciences
By using the ChatGPT API with proprietary algorithms, Dot Compliance’s eQMS delivers real-time, intelligent assistance that not only streamlines workflows but also enhances the compliance of life science organizations. The system’s AI-driven capabilities help QA managers to make informed decisions and enforce good quality management practices, thereby reducing the risk of costly regulatory violations, according to Sitbon.

Doron Sitbon
In recent years, regulatory bodies such as the FDA and EMA have levied millions of dollars in fines on life science companies for violations of quality and safety standards. The use of AI to make informed decisions and enforce good quality management practices can significantly reduce these costs, making a notable impact on the industry’s bottom line, Sitbon explained.
The AI-driven quality management software aims to optimize quality processes, automate tasks, and help professionals in quality assurance focus on mission-critical activities. By seamlessly integrating AI-driven quality management into existing processes, manufacturers can benefit from a system that constantly learns and evolves to offer increasingly accurate insights and predictions.
As the life sciences industry continues to evolve, the need for intelligent assistance in managing complex regulatory demands will only increase, Sitbon predicted. The AI-based eQMS, powered by ChatGPT and other algorithms, could represent a significant step forward in transforming the quality and compliance space. “It’s pretty exciting; it’s almost like science fiction,” Sitbon said.
Filed Under: Data science




Tell Us What You Think!
You must be logged in to post a comment.