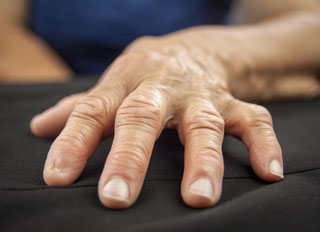
 Daiichi Sankyo Co., Ltd. announced the start of the Phase 3 trial of CHS-0214, an investigational etanercept biosimilar, in rheumatoid arthritis (the RApsody trial) in Japan. Daiichi Sankyo is a co-sponsor of this trial, in collaboration with the U.S. company Coherus BioSciences Inc.
Daiichi Sankyo Co., Ltd. announced the start of the Phase 3 trial of CHS-0214, an investigational etanercept biosimilar, in rheumatoid arthritis (the RApsody trial) in Japan. Daiichi Sankyo is a co-sponsor of this trial, in collaboration with the U.S. company Coherus BioSciences Inc.The trial is a randomized, double-blind, active-control, parallel-group, multicenter, global study, comparing the efficacy and safety of CHS-0214 with Enbrel in subjects with active RA who have demonstrated an inadequate response to methotrexate. The primary endpoint is ACR 20 at 24 weeks. Daiichi Sankyo is developing CHS-0214 in Japan with the intention to enter the biosimilar market in the near future.
Date: August 18, 2014
Source: Daiichi Sankyo
Filed Under: Drug Discovery




