The AI oncology startup ConcertAI recently acquired CancerLinQ, one of the largest oncology real-world data and quality of care technology service entities. Originally developed by the American Society of Clinical Oncology (ASCO) in 2013, CancerLinQ aims to use real-world data and technology to improve cancer care and advance evidence-based research. CancerLinQ has developed one of…
Using AI to unlock new uses for existing cancer medicines
Repurposing is a drug development strategy that has been widely applied in cancer. This strategy, sometimes called label expansion, involves obtaining FDA approval to market a drug for the treatment of new indications, alone or in combination with other drugs. Not only can this approach extend the window of patent protection for a commercialized drug,…
How Lantern Pharma and Code Ocean partnered on oncology drug development
A vision for data-driven drug development in oncology When Peter Carr, principal software architect of Lantern Pharma, stepped into his full-time role in September 2020, the company was on the cusp of a transformation. While AI had been a focus for a number of years, a fresh infusion of cash provided a possibility of expanding…
A glimpse at Big Pharma’s upper echelon in the first three quarters of 2023
In 2023, demand for GLP-1 receptor agonists such as Lilly’s tirzepatitde and Novo Nordisk’s semaglutide surged just as demand for COVID-19 therapies waned. As a result, Novo Nordisk had 33% growth at constant exchange rates over the first nine months of the year. Similarly, Lilly experienced a 37% jump in revenue in the third quarter,…
Insilico Medicine taps AI to nominate small molecule inhibitor ISM9274 as a preclinical cancer therapy
Clinical-stage AI company Insilico Medicine has nominated a novel small molecule inhibitor known as ISM9274 as a preclinical candidate for cancer treatment. The company used its PandaOmics AI platform to analyze genomic data from more than 90 tumor types and identified CDK12 as a promising target for multiple cancers including triple-negative breast cancer, lung cancer,…
Click chemistry breakthroughs drive Shasqi and J&J cancer alliance
In June 2023, the click chemistry-focused startup Shasqi revealed a research pact with Johnson & Johnson Enterprise Innovation. More recently, the company announced that it had expanded the research alliance, furthering work on its intratumorally injected biopolymer, known as SQL70. The collaboration will also apply its clinically validated Click Activated Protodrugs Against Cancer (CAPAC) technology…
Nobel-connected startup Shasqi deepens J&J partnership on CAPAC platform
San Francisco-based oncology startup, Shasqi, announced an expansion of its research collaboration with Johnson & Johnson Enterprise Innovation. The partnership centers on Shasqi‘s CAPAC platform, which is an abbreviation for Click-Activated Protodrugs Against Cancer. The platform separates tumor-targeting from the actual drug payload with the aim of maximizing potency while minimizing toxic side effects. Shasqi’s…
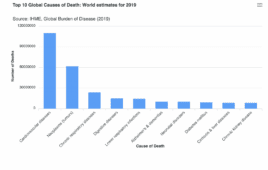
Data-driven insights into the leading causes of death, including cardiovascular disease and cancer
Data sourced from Our World in Data Cardiovascular disease and cancer remain the leading causes of death in the world, based on an analysis of data sources ranging from World Health Organization and Our World in Data. While respiratory illnesses were the third most common cause of death in 2019, deaths in this category…
Johnson & Johnson pharma rebrand highlights innovation as a pillar to reinforce trust
Global pharma and medical device giant Johnson & Johnson (J&J) has ditched its iconic cursive logo that dates back to the late 19th century, and rebranded its Janssen pharma division as Johnson & Johnson Innovative Medicine. The move underscores the company’s push to prioritize higher-margin prescription drugs. This strategic move comes amidst a backdrop of…
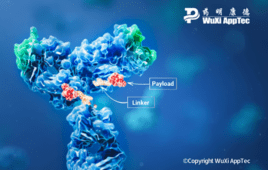
Exploring future cancer therapies: Designing linkers to increase ADC efficacy and reduce toxicity
Antibody-drug conjugates (ADCs) represent a significant paradigm shift in cancer treatment, marrying the precise target recognition of monoclonal antibodies with the potent cell-killing capabilities of cytotoxic agents. In contrast to traditional small-molecule therapies, ADCs are multifaceted structures with three distinct components — i.e., antibody, cytotoxic payload and a linker — each playing a crucial role…
Mapping the cancer patient journey with liquid biopsy
According to the American Cancer Society, one in two men and one in three women will be diagnosed with cancer at some point in their lives. Patients seek treatment to shrink their tumors and ideally achieve remission; however, there is no one-size-fits-all approach. At its core, cancer is a genetic disease: Different types of cancer that affect…
Navigating the future of cell therapies with bit.bio exec Kathryn Corzo
Her previous roles include a stint as the head of development for oncology cell therapies at Takeda. She also led the North American Innovation Center and R&D Digital Accelerator at Sanofi and held a leadership role in Lilly’s oncology business unit. Bit.bio’s differentiated approach Corzo’s decision to join bit.bio was rooted in the company’s pioneering…
How synthetic data accelerates oncology research and drug development
Synthetic data in oncology is transforming how researchers and developers approach real-world evidence. They often need this evidence to test hypotheses, predict outcomes and develop algorithms. But privacy constraints and access related to patient data can create delays and lengthen project timelines. Oncology drug researchers and developers have recently begun using synthetic data in oncology…
10x Genomics’ Chromium platform sheds light on CAR T-cell therapy persistence
Therapy persistence is a vital factor in determining the success of CAR-Ts for blood cancers like leukemia. While CAR-Ts hold great promise for blood cancers such as leukemia, in some cases, the durability of the treatment falls short, leading to a potential relapse. A positive study from researchers at University College London, Great Ormond Street…
AbbVie and Janssen receive positive CHMP opinions for novel cancer therapies
The European Medicines Agency (EMA) could soon change the face of cancer treatment with its potential approval of two novel bispecific antibodies, epcoritamab and talquetamab. The EMA’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting conditional marketing authorization for two novel cancer therapies — AbbVie’s epcoritamab and Janssen’s talquetamab. Epcoritamab for relapsed/refractory…
Decoding the future of RNA vaccines with Aldevron’s Venkata Indurthi
From tackling the COVID-19 pandemic to paving the way for future global health challenges, RNA vaccines have rapidly gained attention in recent years. Researchers are already working to extend their capabilities for next-gen medicines. For example, scientists are working to create multivalent RNA formulations that can fight multiple virus variants. They are also exploring AI…
This biodegradable brain implant delivers cancer-treating drugs
Researchers say they developed a biodegradable brain implant capable of helping to deliver chemotherapy drugs directly to tumors. Medscape News reported that the research marks another step toward using ultrasound to combat cancer. According to the team, led by Thanh Nguyen, these drugs can penetrate the blood-brain barrier to reach these brain tumors. Nguyen serves…
iBio’s chief reveals strategy behind AI-driven bispecific antibody discovery plans
Biotech firm iBio (NYSEA:IBIO) has incorporated EngageTx, a machine learning-driven technology, into its development roadmap. This T-cell engaging antibody panel assists in generating bispecific antibodies targeting cancer cells. In particular, the firm is developing a novel Trophoblast Cell Surface Antigen 2 (TROP-2) bispecific molecule to target TROP-2-positive cancers. In addition to their focus on oncology,…
AdAPT-001 oncolytic adenovirus shows promising phase 1 cancer treatment results
Oncolytic adenoviruses have won significant attention in recent years as a novel approach to cancer treatment. One example of the trend is AdAPT-001 TGF-ß Trap, an engineered variant of the common cold virus equipped with a transforming growth factor-beta (TGF-β) “trap.” This mechanism is designed to latch onto and neutralize TGF-β, an immunosuppressive cytokine involved…
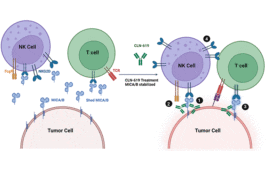
CLN-619 antibody therapy offers a new hope for patients with advanced solid tumors
Boston-based Cullinan Oncology has unveiled data for its new monoclonal antibody therapy, CLN-619, ahead of the American Society of Clinical Oncology (ASCO) 2023 meeting scheduled for June 2–6 in Chicago. The drug could potentially offer a new treatment option for patients with advanced solid tumors. Dr. Judy Wang, Associate Director of Drug Development at the…
Insilico Medicine wins IND approval for AI-designed USP1 inhibitor for cancer trials in U.S. and China
Insilico Medicine has made a significant breakthrough with its AI-designed USP1 inhibitor, ISM3091. The US Food and Drug Administration (FDA) has accepted Insilico’s Investigational New Drug (IND) application for this promising drug, marking a significant milestone for AI-assisted drug discovery. “The FDA’s acceptance of our IND for ISM3091 signifies that the FDA recognizes its potential…
Pharma M&A resurgence: An overview of recent deal-making trends
After something of a slow year in 2022, mergers and acquisition (M&A) activity is ramping back up in the pharma and biotech sector. There have been several especially large M&A deals in the past few months. Examples include Pfizer’s (NYSE:PFE) $43 billion acquisition of Seagen, and Merck’s (NYSE:MRK) $11 billion purchase of Prometheus Biosciences. In December…
The 50 best-selling pharmaceuticals of 2022: COVID-19 vaccines poised to take a step back
The COVID-19 pandemic has had a profound impact on the best-selling pharmaceuticals, leading to shifts in the list with Pfizer and BioNTech’s Comirnaty surpassing AbbVie’s Humira for the No. 1 spot in 2021. That momentum continued in 2022, with Pfizer and BioNTech jointly raking in $59.1 billion in revenue from the sales of the COVID-19…
Lonza and ABL Bio partner on bispecific antibody development for immuno-oncology and neurodegenerative diseases
Lonza (LON:0QNO) and South Korea-based ABL Bio (KOSDAQ:298380) have joined forces to accelerate bispecific antibody development for immuno-oncology and neurodegenerative diseases. Over email, Basel, Switzerland–headquartered Lonza noted that its experience with novel molecular formats enables the company to handle ABL Bio’s unique challenges. In the partnership, Lonza will provide submission-ready chemistry, manufacturing and controls (CMC) data…
AbbVie and Janssen voluntarily withdraw Imbruvica from accelerated approvals for MCL and MZL
AbbVie (NYSE:ABBV) and Janssen Pharmaceutical (NYSE:JNJ) have revealed their intent to voluntarily withdraw the accelerated approvals for Imbruvica (ibrutinib) for patients with mantle cell lymphoma (MCL) and marginal zone lymphoma (MZL) in the U.S. Ibrutinib is a selective Bruton’s tyrosine kinase (BTK). The main reasons for the move relate to FDA’s request for additional studies…