Ozempic, Rybelsus and Wegovy have transformed the diabetes and weight loss treatment landscape, but when it comes to the impact of their active ingredient, semaglutide, on fetal development, “the answer is we do not know,” said Dr. Marijane Hynes, clinical professor of medicine at the George Washington University School of Medicine and Health Sciences. Hynes…
Can payers afford the new era of GLP-1 drugs? Or can they afford not to?
The latest crop of glucagon-like peptide-1 (GLP-1) agonists, such as semaglutide and tirzepatide (technically, a dual GIP/GLP-1 receptor agonist), have upended the way we treat metabolic disorders, including obesity. These drugs mimic gut hormones, improving blood sugar control and often leading to significant weight loss. But as Kyasha Sri Ranjan, Ph.D., engagement manager at Lifescience…
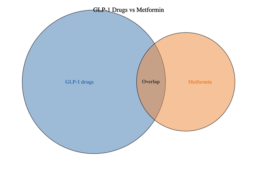
GLP-1s overtake metformin in metabolic clinical trials by a wide margin: A visual exploration
A recent review of more than 2,000 studies related at least indirectly to obesity on clinicaltrials.gov highlights the pronounced significant shift in research focus toward GLP-1 receptor agonists for a range of indications with at least some involvement of metabolic disorders. The site, which provides a robust but not-exhaustive snapshot of clinical trial activity, cites…
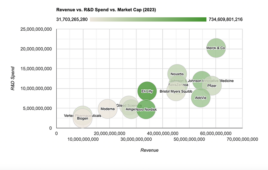
When size doesn’t matter: How Lilly and Novo Nordisk are outperforming pharma giants in value creation
The graphic above shows darker bubbles for companies with larger market caps as of March 25, 2024. Revenue and R&D spending figures are in USD. More data are available when hovering over a given company. After a COVID-19 rebound in public perception and willingness to explore novel modalities, the pharma industry faces mounting pressures…
Could Wegovy’s cardiovascular label expansion be a catalyst for GLP-1 obesity drug coverage?
The recent FDA approval of a cardiovascular risk reduction indication for Wegovy (semaglutide) could point toward a significant opportunity for pharma companies seeking to reshape payer perceptions and expand coverage for next-gen metabolic therapies. This regulatory shift, allowing Wegovy to be prescribed for reducing the risk of major adverse cardiovascular events such as heart attack…
In 2023, Roche and Novartis led the pack in drug pipeline scale
When reviewing R&D spending trends for 2023, Merck & Co. is a clear outlier given its decision to count its $10.3 billion Prometheus acquisition as an R&D charge. In all, the company committed more than half of its revenue to R&D. But Swiss giants Roche and Novartis remain frontrunners in terms of their pipeline of…
How Novo Nordisk’s Wegovy cardiovascular benefits could drive further growth
Semaglutide was already one of the best-selling drugs of recent memory. And Novo Nordisk the fastest-growing Big Pharma firm. Now, the FDA’s decision to expand the label of its weight-loss version of the drug to include cardiovascular benefits could help unlock more growth momentum for Novo Nordisk. This positions Wegovy as the first weight-loss medication…
Novo Nordisk achieves 74% growth surge, clinching fastest-growing pharma title
No longer just a diabetes company, Novo Nordisk’s semaglutide-based therapies have fueled its rise to pharma powerhouse status. From 2021 to 2023, the company cemented its position as the fastest-growing Big Pharma player, reaching a 35% year-over-year growth rate in 2023 and 74% growth from 2020 to 2023 when measured in U.S. dollars. While Pfizer…
GLP-1s, ADCs, AI and the future of pharma
Pharma’s potential breakthroughs in AI, ADCs, and GLP-1 receptor agonists raise a critical question: can innovation outpace the relentless rise of chronic disease? The IQVIA Institute for Human Data Science sheds light on this theme, among many others, in its 80-page Global Trends in R&D 2024 report. Pillar 1: GLP-1 receptor agonists targeting metabolic disease…
Amidst empty labs, signs of biotech’s resurgence emerge
In 2023, a year of accelerated regulatory success, a significant number of biotech labs sat empty in major hubs like San Francisco and Boston. The FDA approved 55 novel therapies in 2023, including Leqembi for early Alzheimer’s and Zurzuvae for postpartum depression. The approval number marked the second highest count in three decades (see graph…
Beyond diabetes and obesity: Can GLP-1 therapies also transform chronic disease treatment?
Glucagon-like peptide-1 (GLP-1) receptor agonists like semaglutide and tirzepatide have cemented their status as two of the most successful drugs in recent memory. Recent projections have estimated that the drug class could fetch $44 billion by 2030 and $71 billion by 2032. But GLP-1 sales could potentially reach greater heights as these therapies move beyond…
Beyond GLP-1: OrsoBio targets mitochondria to treat metabolic disorders from a new and potentially complementary angle
Emerging from stealth in late 2022, Palo Alto–based biopharma startup OrsoBio announced four development programs targeting severe metabolic disorders. The CEO and founder, Dr. Mani Subramanian and chief medical officer and head of development Dr. Rob Myers formerly worked at Gilead. Founded in 2020 with a quest to “try to modulate energy metabolism in severe…
Could an ‘extreme’ hibernating ground squirrel unlock new obesity treatments?
In late 2023, Eli Lilly, whose stock is now up close to 80% over the past year, inked a deal with the Emeryville, California–based Fauna Bio potentially worth $494 million that focuses on the discovery of novel drug targets for treating obesity. In 2020, Fauna entered into an obesity-focused collaboration with Novo Nordisk, Lilly’s primary…
Zepbound helps people lose 25% of body weight on average 88 weeks, but weight regain is a concern
Lilly’s hot weight loss drug Zepbound (tirzepatide) may be one of the most effective drug therapies for weight loss. In an open-label 36-week study, participants lost an average of 20.9% of their body weight in 36 weeks. With an average weight of 107.3 kg (236.5 lbs), that equates to about 22.4 kg (49.4 lbs) on average.…
Tirzepatide beats semaglutide 3-to-1 for weight loss goals in real-world data
A new real-world study (pre-print) is the first to directly compare weight loss outcomes between the popular diabetes medications Lilly’s Mounjaro (tirzepatide) and Novo Nordisk’s Ozempic (semaglutide). The results show Mounjaro users are significantly more likely to achieve meaningful weight loss. Analyzing data from more than 40,000 patients from a large U.S. health database, the…
Lilly’s Zepbound to enter the weight management market with competitive pricing
Lilly’s tirzepatide notched an FDA approval for chronic weight management, potentially clearly the way for billions in additional sales. Analysts have projected that the drug could fetch $26 billion in annual sales by 2030, with roughly two-thirds of that sum related to obesity treatment. Bank of America analyst Geoff Meacham is even more optimistic, predicting…
Five pharmaceuticals featured on Time’s list of top inventions alongside other medical breakthroughs
Time magazine’s most recent roundup of 200 inventions included an array of product types, spanning household gadgets and AI applications. Five distinct pharmaceuticals also made the list, not counting a shipping container for biologics and a vaccine for bees. Among the notable pharmaceutical advances on the list are a novel postpartum depression drug, a novel…
A glimpse at Big Pharma’s upper echelon in the first three quarters of 2023
In 2023, demand for GLP-1 receptor agonists such as Lilly’s tirzepatitde and Novo Nordisk’s semaglutide surged just as demand for COVID-19 therapies waned. As a result, Novo Nordisk had 33% growth at constant exchange rates over the first nine months of the year. Similarly, Lilly experienced a 37% jump in revenue in the third quarter,…
Eli Lilly’s Mounjaro sees explosive sales growth, yet demand outpaces supply
Eli Lilly’s diabetes medication Mounjaro (tirzepatide) is seeing explosive sales growth, jumping from $187.3 million in the third quarter of 2022 to $1.4 billion in the same period this year. Conversely, sales of Lilly’s Trulicity, another diabetes drug, have declined, dipping from $1.85 billion in Q3 2022 to $1.67 billion in Q3 2023. Mounjaro is…
Will GLP-1 drugs transition from obesity and diabetes to diverse clinical indications?
The explosive sales growth of GLP-1 drugs has analysts projecting that the antiobesity drugs could be a $44 billion market by 2030. Some observers are more upbeat, projecting that the sector could be worth more than $100 billion in the coming years. Pfizer CEO Albert Bourla projects that the market will reach $90 billion by…
GLP-1 drug tirzepatide shines in SURMOUNT-3 trial with weight loss of 26.6%
In the phase 3 SURMOUNT-3 trial, tirzepatide recipients saw some of the most impressive weight loss results among trials of GLP-1 drugs, including most notably semaglutide. In the study, participants’ total mean weight loss was 26.6% over 84 weeks following a 12-week intensive lifestyle intervention and subsequent tirzepatide treatment. In all, participants who received tirzepatide…
Eli Lilly’s obesity focus helps propel promising 2023
2023 is shaping up to be a blockbuster year for Eli Lilly. With the company’s stock soaring by almost 54% since the beginning of the year, analysts are largely upbeat about Lilly’s expanded focus on obesity and diabetes treatments. While the company has developed insulin for a century, the company is now broadening its horizons…
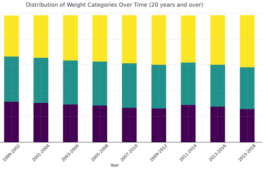
An overview of the GLP-1 landscape in obesity therapeutics
The GLP-1 drug market continues to boom lately thanks to highly effective new medications like Novo Nordisk’s Wegovy and continued demand for the drug class in diabetes. The runaway success of Wegovy and Ozempic (both formulations of semaglutide) has caused Novo Nordisk’s share price to skyrocket in 2023, briefly making the Danish pharmaceutical company the most…
The weight-loss drug market: overhyped or justified?
The obesity drug market has seen a surge of interest recently, largely thanks to the popularity of glucagon-like peptide-1 (GLP-1) drugs like Novo Nordisk’s Wegovy and Eli Lilly’s Mounjaro. The explosion in interest in the drug class has fueled the stock prices of Novo and Lilly, which both have multiple GLP-1 drugs in their portfolios. …
Anti-obesity drugs to command a $44 billion market by 2030
As obesity rates continue to soar across the globe, analysts anticipate that the market for anti-obesity drugs will skyrocket in the coming years. Goldman Sachs projects the market to be worth $44 billion by 2030. That’s an almost 16-fold expansion from its valuation of approximately $2.82 billion in 2022. Barclays is even more upbeat on…