Sanofi revealed that its investigational BTK inhibitor rilzabrutinib notched a significant win in the LUNA 3 phase 3 study, hitting the primary endpoint of durable platelet response in adults with persistent or chronic immune thrombocytopenia (ITP). The study showed a significantly higher proportion of rilzabrutinib-treated patients achieved the platelet response goal compared to placebo in…
After withdrawal, GSK’s Blenrep shows promise in phase 3 DREAMM-7 study
In November 2022, GSK began the process of withdrawing Blenrep’s U.S. marketing authorization after the phase 3 DREAMM-3 trial failed to show an overall survival benefit compared to standard therapy. But GSK has signaled its hope that the B-cell maturation antigen (BCMA) antibody-drug conjugate could have a new lease on life with new data from…
Core trends in 2023 FDA drug approvals: Oncology, neurology and hematology dominate
2023 was a big year for hematology, neurology and oncology, with the medical specialties seeing the most FDA approvals. In terms of sponsors, Pfizer had the most approvals with six total, followed by UCB and Chiesi, each with three apiece. When looking at commercial prospects, AstraZeneca’s respiratory syncytial virus antibody Beyfortus could be the biggest…
Pfizer hemophilia drug marstacimab accepted for FDA, EU review
U.S. and European regulators have accepted applications for Pfizer’s marstacimab, an investigational treatment for hemophilia A and B. The FDA set a decision date in late 2024 while the EMA set the stage for a possible approval in early 2025. The applications are based on positive data from the phase 3 BASIS trial, presented over…
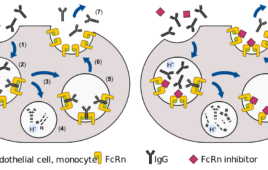
The multi-billion dollar promise of efgartigimod and the broader FcRn inhibitor market
Thanks to promising candidates such as efgartigimod, the Fc receptor (FcRn) inhibitor market is poised to reshape the autoimmune disease treatment landscape. Sales projections for the market top $10 billion, as Driehaus Capital Management estimated in 2020. The addressable U.S. patient base spans more than 228,500 individuals across various conditions including myasthenia gravis, warm autoimmune…
Sanofi ALTUVIIIO hemophilia A treatment: FDA accepts sBLA based on pediatric phase 3 data
The FDA has accepted Sanofi’s supplemental Biologics License Application (sBLA) for ALTUVIIIO, a novel, high-sustained factor VIII replacement therapy for adults and children with hemophilia A. The FDA based the decision on positive final data from the pivotal Phase 3 XTEND-Kids trial in children under 12 with hemophilia A. ALTUVIIIO (efanesoctocog alfa) is the first…
New study sheds light on Eliquis and Xarelto switching
For patients with an elevated stroke risk, switching anticoagulant medications could be a health gamble. Those who change from Eliquis (apixaban) to Xarelto (rivaroxaban) could face almost double the risk of stroke or severe bleeding. Conversely, sticking with Eliquis or transitioning from rivaroxaban to Eliquis appears to be a safer option. Those are key takeaways…
AbbVie and Janssen receive positive CHMP opinions for novel cancer therapies
The European Medicines Agency (EMA) could soon change the face of cancer treatment with its potential approval of two novel bispecific antibodies, epcoritamab and talquetamab. The EMA’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting conditional marketing authorization for two novel cancer therapies — AbbVie’s epcoritamab and Janssen’s talquetamab. Epcoritamab for relapsed/refractory…
XOMA acquires Ixinity’s commercial payment and milestone rights in $9.6M
Biotech royalty aggregator XOMA (Nasdaq:XOMA) has acquired the commercial payment and part of the milestone rights to Ixinity (trenonacog alfa), a hemophilia B therapy originally from Aptevo Therapeutics (Nasdaq:APVO). The aggregator expects the move to bolster its royalty and milestone portfolio, potentially providing consistent cash flow and ramping up its royalty aggregation strategy. The acquired…
At ASH, Genentech showcases expanded hematology portfolio that includes bispecific antibodies
At ASH 2022, Roche (SWX: ROG) subsidiary Genentech presented new data demonstrating the potential of glofitamab and mosunetuzumab, its investigational CD20xCD3 T-cell engaging bispecific antibodies, as fixed-duration, off-the-shelf treatment options for two types of non-Hodgkin’s lymphoma (NHL) – large B-cell lymphoma (LBCL) and follicular lymphoma (FL), respectively. “The new data demonstrated durable and impressive patient…
Rilzabrutinib study highlights cognitive impairment in patients with chronic immune thrombocytopenia
A Phase 1/2 study (NCT03395210) investigating Sanofi (Nasdaq:SNY) Bruton tyrosine kinase inhibitor rilzabrutinib in immune thrombocytopenia (ITP) has also shed light on the cognitive impairment involved in the disease. Patients with ITP, an autoimmune disease that involves increased platelet destruction, have often complained of fatigue, problems with cognition and other symptoms that physicians have dismissed,…
Iptacopan from Novartis could become preferred therapy for paroxysmal nocturnal hemoglobinuria
Novartis (NYSE: NVS) recently announced that the pivotal iptacopan Phase 3 APPLY-PNH trial met both its primary and most secondary endpoints in patients with paroxysmal nocturnal hemoglobinuria (PNH). The company recently presented the data at this year’s American Society of Hematology (ASH) meeting. Almost all iptacopan recipients had blood-transfusion independence and had clinically meaningful patient-reported-fatigue improvements. There…
Monoclonal antibody sutimlimab did not interfere with COVID-19 vaccination response in Phase 3 studies
The pandemic has complicated the treatment of cold agglutinin disease (CAD), a rare autoimmune hemolytic anemia accounting for roughly 20% of all autoimmune hemolytic anemia. CAD affects older adults primarily. The anti-CD20 therapies commonly used off-label to treat the disease also increase the risk of serious COVID-19 infections and interfere with the immune response to COVID-19…
Hemophilia gene therapy Hemgenix sets record for world’s most expensive drug
FDA has approved CSL Behring’s (ASX: CSL) Hemgenix (etranacogene dezaparvovec), an adenovirus-associated virus–based gene therapy for adults with hemophilia B. The price tag for the gene therapy — the first for hemophilia B — is $3.5 million. The drug is administered as a single infusion. Hemophilia B is a genetic bleeding disorder caused by missing or…
Why GSK is pulling multiple myeloma drug Blenrep from U.S. market
FDA has requested that GSK plc (LSE/NYSE:GSK) withdraw the relapsed and refractory multiple myeloma drug Blenrep (belantamab mandolin-blmf) from the U.S. market. GSK announced in a statement that it has initiated the process. On November 7, the company announced that the drug did not meet its primary endpoint of progression-free survival (PFS) in the Phase 3 DREAMM-3…
Pfizer finalizes acquisition of Global Blood Therapeutics
In August, Pfizer (NYSE:PFE) announced its plan to acquire Global Blood Therapeutics (GBT) for $68.50 per share, or roughly $5.4 billion. Today, the company announced that it had finalized the acquisition, giving it access to GBT’s portfolio of drug candidates for sickle cell disease (SCD). The GBT acquisition also adds Oxbryta (voxelotor), a novel sickle…
Kyowa Kirin is targeting disparities in African Americans with rare blood cancer
Kyowa Kirin North America (KKNA) is working to address the racial disparities affecting the care and outcomes of African American patients with Cutaneous T-cell Lymphoma (CTCL). CTCL is a rare form of blood cancer that first appears on the skin and is often mistaken for more common dermatologic conditions. CTCL can affect the skin as…
Merck wins Fast Track designation from FDA for experimental anticoagulant therapy
FDA has informed Merck (NYSE:MRK) that its investigational anticoagulant therapy MK-2060 has received Fast Track designation. The program accelerates the review of new drugs to treat serious conditions or unmet medical need. The Fast Track designation covers the risk reduction of major thrombotic cardiovascular events in patients with end-stage renal disease (ESRD). “We are encouraged…
Roche shares upbeat Phase 3 data for Hemlibra in hemophilia
Facing potential competition from Novo Nordisk’s (NYSE:NVO) investigational blood coagulation factor stimulants Mim8, Roche (SIX:RO, ROG; OTCQX:RHHBY) released positive new data from the Phase 3 HAVEN 6 study focused on the use of Hemlibra (emicizumab) in patients with mild or moderate hemophilia A. Hemlibra won FDA approval in 2018 for hemophilia A without factor VIII…
Akebia Therapeutics gets complete response letter from the FDA for vadadustat for CKD-associated anemia
The biopharma Akebia Therapeutics (Nasdaq:AKBA) has received a dreaded complete response letter from FDA for vadadustat, the experimental oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor proposed as a treatment for anemia associated with chronic kidney disease (CKD). A complete response letter communicates the agency’s rejection of an application in its present form. In the case…
Mitapivat from Agios scores FDA nod as for hemolytic anemia in adults
Agios Pharmaceuticals Inc. (NASDAQ:AGIO) has announced that Pyrukynd (mitapivat) has won FDA approval to treat hemolytic anemia in adults with pyruvate kinase (PK) deficiency. A first-in-class oral PK activator, mitapivat is the first disease-modifying therapy for hemolytic anemia to win FDA approval. FDA based its decision on data from the Phase 3 ACTIVATE and ACTIVATE-T…
What is Human AB serum?
By Trevor Smith, MS MBA, Product Manager, Immune Cells Human AB Serum is a staple in the biological research field, providing nutrients, vitamins and necessary growth factors in cellular culture and reliable controls in in vitro diagnostics. To truly understand serum, it is important to learn about its different production and processing methods. Starting material…
Adakveo: An FDA-indicated treatment for sickle cell disease
Adakveo (crizanlizumab-tmca) from Novartis is FDA indicated for reducing the number of vaso-occlusive crises in people aged 16 years and older with sickle cell disease. Novartis describes it as “the first and only once-monthly medication to reduce the number of pain crises in sickle cell disease.” The drug works by inhibiting selectin, a group of carbohydrate-binding transmembrane molecules…
Xospata met primary endpoint in confirmatory trial
Xospata (gilteritinib) from Astellas Pharma is indicated for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia with a FMS-like tyrosine kinase 3 (FLT3) mutation as detected by an FDA-approved test. In March, Astellas announced that the drug met its primary endpoint in a planned interim analysis of a Phase 3 confirmatory…
FDA advisory committee recommends not approving FibroGen’s anemia drug candidate roxadustat
The FDA’s independent Cardiovascular and Renal Drugs Advisory Committee (CRDAC) voted against approving Roxadustat from FibroGen, which would be a novel treatment of anemia resulting from chronic kidney disease (CKD). Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor. FibroGen’s CEO said in a statement that the company believes scientific evidence warrants approval of…