AbbVie has shared new efficacy and safety data for Rinvoq (upadacitinib) in adults and adolescents with atopic dermatitis (AD) from a trio of ongoing phase 3 studies. Spanning 140 weeks, these studies sustained the co-primary endpoints of Eczema Area and Severity Index 75 (EASI 75) and validated Investigator’s Global Assessment for Atopic Dermatitis 0/1 (vIGA-AD…
Legacy Healthcare aims to upend alopecia areata treatment with a botanical drug
Imagine a world where botanical drugs could shape the future of medical treatment. Saad Harti, CEO of Swiss company Legacy Healthcare, doesn’t just imagine it; he’s on a mission to make it a reality. With an initial focus on alopecia areata in children and adolescents, Legacy Healthcare believes the plant extract Coacillium could be a…
FDA places clinical hold on Sun Pharma’s dermatology drug as a result of blood clot risk
Sun Pharmaceuticals (NSE:SUNPHARMA) has received a clinical hold from the FDA on its experimental dermatological drug deuruxolitinib over the potential for thromboembolic incidents. The hold pertains to patients taking a 12-mg dose of the Janus kinase (JAK) inhibitor. Reuters was the first to report the news. Sun Pharma maintains confidence in deuruxolitinib despite FDA clinical…
FDA approves Pfizer’s Cibinqo for adolescent atopic dermatitis
Pfizer (NYSE:PFE) has announced that the FDA has approved its supplemental New Drug Application (sNDA) for Cibinqo (abrocitinib) for moderate-to-severe atopic dermatitis. The approval expands the indication for the drug, which was previously only approved for adults with the skin condition in early 2022. The expanded indication includes adolescents between the ages of 12 and…
Positive results for oral difelikefalin in Phase 2 nostalgia paresthetica study
NEJM has published positive results from the KOMFORT Phase 2 trial of oral difelikefalin for nostalgia paresthetica, a condition involving itching, burning or tingly sensations on the back. Cara Therapeutics (Nasdaq:CARA) is the developer of the drug, which won FDA approval in an injectable form for moderate-to-severe pruritus in hemodialysis patients in 2021. That form…
New study suggests link between Moderna COVID-19 booster and chronic hives
A Swiss study found that the monovalent Moderna (Nasdaq:MRNA) COVID-19 booster vaccine may be linked to a higher risk of a type of hives known technically as chronic spontaneous urticaria (CSU). In a cohort of the study based in the Swiss canton of Vaud, 90% of people who received the mRNA-based Moderna COVID-19 booster and developed…
Lilly’s lebrikizumab yielded durable skin clearance in Phase 3 atopic dermatitis studies
Eli Lilly and Company’s (NYSE:LLY) Phase 3 monotherapy ADvocate trials in atopic dermatitis (AD) found that the investigational interleukin 13 inhibitor lebrikizumab supported durable skin clearance and reduced itching in a subset of patients with atopic dermatitis. The Indianapolis–based company defined responders as those with at least 75% improvement in the Eczema Area and Severity…
AbbVie’s Skyrizi becomes first drug to win FDA nod for Crohn’s disease
AbbVie (NYSE:ABBV) has announced that FDA has approved Skyrizi (risankizumab-rzaa) as the first interleukin-23 (IL-23) inhibitor to treat adults with moderately to severely active Crohn’s disease (CD). Skyrizi is also FDA-approved as a treatment for moderate to severe plaque psoriasis and active psoriatic arthritis. IL-23 inhibitors have surged in popularity recently, given their ability to…
FDA approves Olumiant as first systemic therapy for baldness form
The FDA has announced that it has approved Lilly’s Olumiant (baricitinib) oral tablets to treat adults with severe alopecia areata, a form of baldness resulting from the body attacking its hair follicles. Manifesting as patchy baldness, alopecia areata affects more than 300,000 people in the U.S. The incidence of alopecia areata is growing. “Access to…
FDA approves first microencapsulated benzoyl peroxide for rosacea
Sol-Gel (Nasdaq:SLGL) and Galderma have announced that it has won FDA approval for Epsolay cream, a proprietary topical formulation of benzoyl peroxide, 5%, for treating inflammatory rosacea lesions. Rosacea is a common condition, but experts have struggled to estimate its global prevalence. A 2018 estimate in the British Journal of Dermatology concluded that the condition affects between…
Lilly’s lebrikizumab with topical corticosteroids improved atopic dermatitis in the study
Eli Lilly (NYSE:LLY) has announced promising results from a Phase 3 study of patients with moderate-to-severe atopic dermatitis (AD). At 16 weeks, 70% of patients with moderate-to-severe atopic dermatitis (AD) taking both lebrikizumab and standard-of-care topical corticosteroids (TCS) achieved at least 75% improvement in overall disease severity (EASI-75*) in the ADhere trial. The company made…
Network meta-analysis ranks guselkumab best for skin clearance in psoriatic arthritis
A recent comprehensive network meta-analysis (NMA) concluded that Tremfya (guselkumab) from Janssen (NYSE:JNJ) ranked highest for the overall level of skin clearance among 23 treatment regimens for active psoriatic arthritis (PsA). The NMA drew on data from 33 Phase 3 randomized clinical trials. Of those, 15 were targeted PsA therapies such as the IL-23p19 inhibitors such…
Biogen announces biosimilars collaboration deal with Xbrane
Having recently sold an equity stake in Samsung Bioepis for up to $2.3 billion, Biogen (NSDQ:BIIB) has announced a licensing deal with Xbrane Biopharma (STO:XBRANE), a Swedish biosimilar developer. The agreement relates to the development of Xcimzane, a preclinical monoclonal antibody referencing Cimzia (certolizumab pegol) from UCB. Having first won FDA approval in 2018 for moderate to…
FDA approves Pfizer’s JAK1 inhibitor Cibinqo for moderate-to-severe atopic dermatitis
Pfizer (NYSE:PFE) has announced that the FDA has approved the oral Janus kinase 1 inhibitor Cibinqo (abrocitinib) for adults with refractory, moderate-to-severe atopic dermatitis (AD). In 2020, Pfizer CEO Dr. Albert Bourla projected that Cignqo would generate $3 billion in peak sales. In 2021, SVB Leerink projected the drug would generate $2 billion in sales…
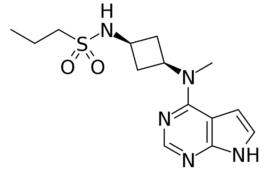
FDA grants Rinvoq new indication for moderate to severe atopic dermatitis
FDA has approved Rinvoq (upadacitinib) from AbbVie (NYSE:ABBV) to treat moderate to severe atopic dermatitis in individuals at least 12 years old. AbbVie anticipates 2025 risk-adjusted sales for Rinvoq to top $7.5 billion. The most common type of eczema, atopic dermatitis, can be managed with various therapies, including corticosteroids, topical calcineurin inhibitors and immunosuppressants. Nevertheless, the…
Incyte wins FDA nod for eczema cream
The FDA has approved Incyte’s (NSDQ:INCY) ruxolitinib, a cream for short-term and non-continuous chronic treatment of mild-to-moderate atopic dermatitis (eczema). As many as 15 million Americans have atopic dermatitis, according to the Cleveland Clinic. In addition, the NIH estimates that the condition affects 30 million Americans, predominantly children and adolescents. The labeling for ruxolitinib (Opzelura) constrains its…
Tremfya bests Cosentyx in psoriasis study
Janssen’s Tremfya (guselkumab) generally had higher efficacy in treating moderate-to-severe psoriasis than Cosentyx (secukinumab) from Novartis, according to recent data published in the Journal of Dermatological Treatment. In the 48-week Phase 3 ECLIPSE study, a greater number of guselkumab recipients achieved at least a 90% and 100% improvement from baseline in Psoriasis Area and Severity…
Pfizer’s abrocitinib goes head-to-head with Sanofi’s Dupixent
Pfizer (NYSE: PFE) recently announced that its once-daily oral Janus kinase 1 (JAK1) inhibitor abrocitinib bested Sanofi’s Dupixent (dupilumab) in a Phase 3 study focused on moderate to severe atopic dermatitis (AD). Meanwhile, Sanofi (EPA:SAN) announced that a Dupixent pivotal trial met its primary and secondary endpoints, making it the first biologic to demonstrate significant…
34 of the most innovative pharmaceutical products
The Galien Foundation has revealed its latest nominees for the 2021 Prix Galien USA Award highlighting innovations in biotechnology, pharmaceutical agents, medical technology and digital health products. Entrants to the competition must have received FDA approval within the past five years and demonstrate exceptional therapeutic potential. The Galien Foundation does not use financial data to…
Why Janssen believes next-gen drug Tremfya can meet unmet needs in psoriasis and beyond
Two decades ago, psoriasis was a poorly understood condition. Treatment options for severe psoriasis included powerful immunosuppressant agents such as methotrexate and cyclosporin that can have significant side effects. But the fact those immunosuppressant drugs were effective at treating psoriasis helped pave the way to use biologics to treat the condition. The experience “really taught…