[Updated April 23, 2024] In the face of rising R&D costs and growing pricing pressures from payers, the pharma and biotech sectors continue to transform to adapt to an evolving landscape. While workforce reductions persist in 2024 for some companies, major players like AbbVie, AGC Biologics, Amgen, Novartis and Thermo Fisher Scientific are demonstrating confidence…
With prices topping $4 million, high stakes define cell and gene therapy landscape
Cell and gene therapies often promise unparalleled treatment options for patients, but sometimes those benefits come at an extraordinary cost. The therapy class is responsible for the world’s most expensive drugs, including the recently FDA-approved gene therapy, Libmeldy, with a wholesale acquisition cost of $4.25 million. The prior record holder, Hemgenix, had a $3.5 million…
Big Pharma clicks soared, but new cell therapies made you buzz: What drove biopharma interest in 2023?
Despite biopharma’s 2023 layoffs and challenges, innovation won your clicks last year with over 130,000 of them on our Pharma 50 report alone. But while giants dominated, your clicks showed disruptive tech wasn’t far behind. The next-most popular article was a roundup of 100 trailblazing cell and gene therapy companies with more than 80,000 views.…
Rice Biotech Launch Pad plans to make Houston a top-tier biotech hub
Houston boasts many world-class assets that have made it a formidable player on the global stage. From the world’s largest medical complex to mission control for the cosmos, few other cities can compete with its diverse strengths. Houston is also home to the prestigious Rice University, renowned for its leading science and engineering programs. Houston,…
Caribou Biosciences’ CEO discusses CRISPR progress, future goals, and gender equality in biotech
Founded in 2011, Caribou Biosciences is a pioneer in the development of CRISPR genome editing technologies, a field honored with the Nobel Prize in Chemistry in 2020. Co-founded by Jennifer Doudna, Ph.D., one of the Nobel laureates, and CEO Rachel Haurwitz, and two other CRISPR pioneers, the company has raised over $800 million in funding,…
Recursion partners with NVIDIA to unveil Phenom-Beta, democratizing access to its $1 Billion phenomics investment
In sync with the JP Morgan Health Care Conference, Recursion Pharmaceuticals has unveiled Phenom-Beta, a deep learning model designed to transform cell microscopy images into meaningful biological representations. “Phenom-Beta allows you to take images of human cells from a microscope or other sources and turn them into mathematical representations of biology,” said Chris Gibson, CEO…
The global biotech funding landscape in 2023: U.S. leads while Europe and China make strides
In 2023, the U.S. continued to demonstrate its position as the biotech funding leader, commanding over one-third, 35%, of the global investment in the sector. Overall, U.S. biotech firms attracted $56.79 billion in funding, according to a survey of Crunchbase data. Next in line was China, which contributed about 12.7% to the global funding pool,…
Kristofer Mussar on VectorBuilder’s ethical imperative in gene delivery
As genetic technologies unlock new possibilities, companies like VectorBuilder aim to navigate progress responsibly, guided by a strong moral compass. “Ethics to me is the one thing that really is so, so important,” said Kristofer Mussar, managing director of VectorBuilder GmbH. “I have such a high ethical threshold I make decisions that are sometimes not…
Unicorn gene-delivery firm VectorBuilder eyes future breakthroughs
Genetic tools are growing more powerful by the day and hold immense medical promise. Kristofer Mussar, managing director of VectorBuilder GmbH who holds a Ph.D. in molecular genetics and epigenetics, noted that in the wake of the pandemic, genetic research requires increased awareness and ethical stewardship to responsibly tap into its power. While the current…
Unlocking the secrets of cellular immunogenicity: A deep dive in ELISpot assays
Next generation therapies and vaccine research have recently dominated headlines and scientific discovery. This research and its ability to treat previously untreatable conditions has rightfully excited the scientific and medical communities worldwide. But the complexities involved require a nuanced approach to ensure the treatment is safe and effective. Integrating cell culture into cellular immunity testing…
Prix Galien Awards: The most innovative biotech, pharma, and orphan drugs of 2023
Prix Galien names 2023 winners in pharma and biotech In the world of medical innovation, few accolades carry as much weight as the Prix Galien Awards, which highlight the advances in biotech, pharmaceuticals, and other domains. The 2023 winners include Bristol Myers Squibb’s Camzyos (mavacamten) as the best biotechnology product and Lilly’s Mounjaro (tirzepatide) and…
Pfizer Ignite: Kathy Fernando’s vision for accelerating biotech innovation
Kathy Fernando, the senior vice president, head of Pfizer Ignite and Pfizer CentreOne, has had a professional trajectory marked by pivotal serendipities. One occurred when attending a seminar at the University of Pennsylvania, where she met Dr. Drew Weissman, a prominent immunologist and RNA vaccine researcher. Weissman, along with Katalin Karikó, recently received the Nobel…
Biotech sees new shoots of growth but has thorns left to prune
Today, Boston-based Ginkgo Bioworks’ recently unveiled a deal with Pfizer on RNA-based drug candidates, reflecting the growing interest in RNA therapies. Additionally, Carlsbad, California–based Ionis Pharmaceuticals inked a pact with Roche focused on RNA-targeted therapies for Alzheimer’s and Huntington’s diseases. This development underscores the resilience of prominent biotech hubs. According to data from real estate…
The balancing act of biotherapy: Be The Match BioTherapies’ Abby Waters on agility and beyond
Professionals in the biotherapy world are engaged in a sort of balancing act, juggling the nuances of product development, complex science, leadership dynamics, and patient-centricity. In a recent interview, Abby Waters, Ph.D., senior manager of solution owners at Be The Match BioTherapies, opens up about her decade-long journey navigating these multifaceted challenges. From embracing agile…
Molecular photoswitches: Illuminating the future of inherited retinal disease treatment
Vision, one of our most valued senses, is a complex orchestration of light-sensing photoreceptors, neurons, and intricate pathways leading to the brain. Central to this native biological system are the photoreceptors – the rods and cones – cells that are responsible for capturing light and initiating visual perception. For many with inherited retinal diseases (IRDs),…
10 rising stars of North American biotech: Cities emerging as life sciences hubs
As the biotech industry matures, several North American cities are emerging biotech hubs, aiming to become the next epicenters of innovation and growth. While traditional hubs like Boston and San Francisco continue to dominate, a new wave of cities is making strides in biotech. For instance, Pittsburgh, an up-and-coming biotech hub, is tapping its biomanufacturing…
Ginkgo Bioworks and Google Cloud forge five-year AI and biology partnership
Founded in 2008, Ginkgo Bioworks’ stock jumped almost 25% on August 29, hitting $2.22, after unveiling a five-year partnership with Google Cloud. The partnership centers around the development of novel AI tools for biology and biosecurity. In particular, Ginkgo hopes to further its mission to make biology easier to engineer in the AI era. Opting…
Cellares teams up with Bristol Myers Squibb to explore automated CAR-T cell therapy manufacturing
Less than a week after announcing that it has secured $255 million in Series C funding, South San Francisco-based startup Cellares has revealed that Bristol Myers Squibb has joined its Technology Adoption Partnership (TAP) program. To date, the company has raised more than $355 million in total financing. As part of the TAP program, Bristol…
Navigating the future of cell therapies with bit.bio exec Kathryn Corzo
Her previous roles include a stint as the head of development for oncology cell therapies at Takeda. She also led the North American Innovation Center and R&D Digital Accelerator at Sanofi and held a leadership role in Lilly’s oncology business unit. Bit.bio’s differentiated approach Corzo’s decision to join bit.bio was rooted in the company’s pioneering…
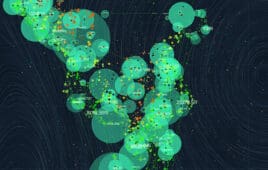
30 promising biotech startups: Scatter plot
The preceding visual representation of the biotech startups of 2023 are grouped — clustered — according to their focus areas. Each cluster color corresponds to a specific domain: Orange for advanced molecular techniques (Cluster 0). Blue for cell and gene therapies (Cluster 1). Green for AI-driven drug discovery (Cluster 2). Red for epigenetics and genomic…
Interactive snapshot: 30 promising biotech startups
2023 is shaping up to be a pivotal year for promising biotech startups. While the sector faced multiple waves of layoffs, a silver lining is evident: the growing maturity of AI and genomics technologies is fueling a new wave of startup advances, especially in drug development. Amidst the backdrop of that turbulence, a select group…
30 biotech startups making waves
The biotech industry is facing a reckoning in 2023. To date, roughly 100 biopharmas have cut workers this year, matching the total number of layoffs in the sector in 2022. Many biotech startups have been hit hard. The wave of job cuts comes on the heels of a biotech boom following the COVID-19 pandemic, when…
10x Genomics’ Chromium platform sheds light on CAR T-cell therapy persistence
Therapy persistence is a vital factor in determining the success of CAR-Ts for blood cancers like leukemia. While CAR-Ts hold great promise for blood cancers such as leukemia, in some cases, the durability of the treatment falls short, leading to a potential relapse. A positive study from researchers at University College London, Great Ormond Street…
Decoding the future of RNA vaccines with Aldevron’s Venkata Indurthi
From tackling the COVID-19 pandemic to paving the way for future global health challenges, RNA vaccines have rapidly gained attention in recent years. Researchers are already working to extend their capabilities for next-gen medicines. For example, scientists are working to create multivalent RNA formulations that can fight multiple virus variants. They are also exploring AI…
The shift from manual to machine learning in cell and gene therapy drug discovery
Cell and gene therapy (CGT) manufacturing is rapidly transitioning from scientific curiosity to clinical reality. But the manufacturing complexity of CGTs far outpaces traditional biologics production, presenting a multifaceted challenge that is part scientific, part technological. “The manufacturing process for cell therapies and gene therapies is infinitely more complex than it is for let’s say,…