With promising interim phase 2 data in hand, Cybin believes psychedelic therapy will become a reality in the “not too distant future,” according to CEO Doug Drysdale. As recently as the 1990s, it would be difficult to imagine that a psychedelic drug would potentially be a clinical option for a mood disorder like depression. But…
Alzheimer’s at an inflection point as drug and diagnostics breakthroughs emerge
Alzheimer’s disease research appears to be hitting its stride, thanks to recent therapeutic advances in drug development and the emergence of biomarkers to detect the condition. “All the pieces of the puzzle of precision medicine, which is already quite common in oncology, are now in place,” said Hartmuth Kolb, vice president, neuroscience biomarkers and R&D…
Analysis shows that cariprazine may have cost savings potential for treating MDD
Vraylar (cariprazine), which recently scored FDA approval as an adjunctive treatment for Major Depressive Disorder (MDD), could have positive implications for healthcare resource utilization. A new analysis suggests that this atypical antipsychotic, initially developed by Allergan (now AbbVie), could curb healthcare spending of MDD treatment. In the economic analysis, patients who received cariprazine as their…
Aiming to tame psychedelics’ wild side in pursuit of FDA approval
Doors of perception: Psychedelic renaissance or Pandora’s box? In one sense, psychedelics have always been divisive in mainstream Western culture. During their heyday in the 1960s, proponents lauded psychedelics’ virtues for psychological healing and exploration. Troubling reports also emerged — stories of bad trips, psychological breaks, and mostly apocryphal yet sensationalized reports of individuals leaping…
Phase 3 trial shows donanemab reduces Alzheimer’s symptoms by 35%
According to results from the Phase 3 TRAILBLAZER-ALZ 2 study published in JAMA, the monoclonal antibody donanemab significantly slowed cognitive and functional decline in patients with early symptomatic Alzheimer’s disease by approximately 35% at one year compared to placebo. The trial enrolled 1736 patients across 277 research centers in 8 countries. Another monoclonal antibody, Leqembi…
Leqembi could mark new era in Alzheimer’s treatment progress: An overview of the evolving drug development scene
Today, the FDA granted traditional approved to lecanemab (branded as Leqembi), a monoclonal antibody from Eisai and Biogen for adult patients with Alzheimer’s disease. The agency made the decision on the basis of a confirmatory trial that showed its clinical benefit. The drug, which reduces the formation of amyloid plaques in the brain, is the…
The Allen Institute is employing AWS Cloud and machine learning to decode brain mysteries
High-resolution mapping of the human brain involves managing and interpreting a colossal amount of data. Shoaib Mufti, the head of data and technology at the Allen Institute for Brain Science, described the organization’s approach to these challenges in a recent interview. The project uses artificial intelligence to analyze millions of data points from brain imaging…
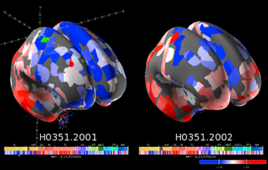
The Brain Knowledge Platform aims to illumine the brain’s cellular universe
Despite substantial progress in brain research, our understanding of the human brain remains limited. “We really don’t understand the fine circuitry of the brain — even in a relatively simple organism like a mouse,” confessed Ed Lein, a senior investigator at the Allen Institute for Brain Science. But AI in neuroscience research promises to change…
Beyond the trip with non-hallucinogenic psychoplastogens in neuropsychiatry
Interest in ketamine and psilocybin as potential therapies for mood disorders has surged since around 2010. A groundbreaking 2000 study at Yale revealed the powerful antidepressant effects of ketamine, a dissociative anesthetic. Unlike traditional antidepressants which can take weeks or months to have an impact, a single dose of ketamine led to significant improvements in…
Natalizumab and PML: The complex dance of benefit and risk for MS
Biogen’s Tysabri (natalizumab), the first humanized monoclonal antibody for multiple sclerosis (MS), sparked optimism among MS patients following its FDA approval in 2004. The drug offered significant benefits, reducing relapses for patients resistant to other treatments. This was a significant milestone in the treatment of MS, but the journey of natalizumab and PML soon took…