[Updated April 4, 2024] In the face of rising R&D costs and growing pricing pressures from payers, the pharma and biotech sectors continue to transform to adapt to an evolving landscape. While workforce reductions persist in 2024 for some companies, major players like AbbVie, AGC Biologics, and Amgen are demonstrating confidence in the industry with…
Microsoft and 1910 Genetics: AI-powered partnership targets billion-dollar savings and growth in drug discovery
The pharmaceutical industry is at a critical juncture: AI and other technological advances offer unprecedented potential, yet the cost of developing new drugs has ballooned for decades, surpassing $2 billion in recent years with the projected return on investment (ROI) falling to a mere 1.2% in 2022, according to Deloitte. Another dimension of the problem…
Amidst empty labs, signs of biotech’s resurgence emerge
In 2023, a year of accelerated regulatory success, a significant number of biotech labs sat empty in major hubs like San Francisco and Boston. The FDA approved 55 novel therapies in 2023, including Leqembi for early Alzheimer’s and Zurzuvae for postpartum depression. The approval number marked the second highest count in three decades (see graph…
Interview: MIT legend Robert Langer backs Lindus Health’s ‘anti-CRO’ strategy
Biotech startup Lindus Health just made a big splash by adding MIT professor and Moderna co-founder Robert Langer, Sc.D., to its advisory board. Lindus aims to shake up the world of clinical trials with its “anti-CRO” approach that promises faster and more reliable trials for life sciences companies. The addition of Langer, described as the…
Rice Biotech Launch Pad plans to make Houston a top-tier biotech hub
Houston boasts many world-class assets that have made it a formidable player on the global stage. From the world’s largest medical complex to mission control for the cosmos, few other cities can compete with its diverse strengths. Houston is also home to the prestigious Rice University, renowned for its leading science and engineering programs. Houston,…
Lumen Bioscience cracks the code on spirulina as a biologics factory for c. diff, metabolic disease and more
Clostridioides difficile, commonly known as C. diff, is a significant health threat in the U.S. Recent estimates suggest that C. diff, a common bacteria, can cause infection in roughly 500,000 patients annually in the U.S., with around 30,000 of these cases resulting in death. “Actually, it’s more like 5 million when you think about it…
Could an ‘extreme’ hibernating ground squirrel unlock new obesity treatments?
In late 2023, Eli Lilly, whose stock is now up close to 80% over the past year, inked a deal with the Emeryville, California–based Fauna Bio potentially worth $494 million that focuses on the discovery of novel drug targets for treating obesity. In 2020, Fauna entered into an obesity-focused collaboration with Novo Nordisk, Lilly’s primary…
50 of the best-funded biotechs of 2023
As the year draws to a close, it is clear that molecular science and diagnostics is the hottest funding area in the biotech industry. In an analysis of 50 of the best-funded biotechs of 2023 focused on human health, molecular and science and diagnostics startups collectively attracting roughly $945 million, dwarfing the figures in other…
AWS expands collaborations with Amgen and Merck to advance AI in drug discovery and manufacturing
At its annual re:Invent event, Amazon Web Services (AWS) announced expanded alliances with two leading drug developers, Amgen and Merck, to create generative artificial intelligence (AI) technologies aimed at accelerating drug discovery and increasing efficiencies in manufacturing processes. Merck has been working with AWS and Accenture for several years whereas Amgen and AWS have collaborated…
Nipocalimab shows promise in RA subgroups in phase 2a IRIS-RA study
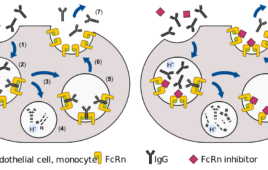
Johnson & Johnson’s nipocalimab, which works by targeting the neonatal Fc receptor (FcRn), has the potential to treat an array of autoimmune conditions. But the antibody recently hit a snag in the first-ever clinical study of an FcRn inhibitor in rheumatoid arthritis (RA), missing its primary endpoint. The development has sparked debate within the rheumatology…
Anticipating IND submission: Ensuring your drug is ready for preclinical toxicology studies
Drug development is often a vast and intricate journey. Each phase signifies an advancement in the process, always with an eye toward patient safety and efficacy. But before any therapeutic finds itself on the bedside tables of hopeful patients, it faces a formidable challenge: preclinical toxicology testing. As the gateway to clinical trials, this early…
Unlocking the secrets of cellular immunogenicity: A deep dive in ELISpot assays
Next generation therapies and vaccine research have recently dominated headlines and scientific discovery. This research and its ability to treat previously untreatable conditions has rightfully excited the scientific and medical communities worldwide. But the complexities involved require a nuanced approach to ensure the treatment is safe and effective. Integrating cell culture into cellular immunity testing…
Five pharmaceuticals featured on Time’s list of top inventions alongside other medical breakthroughs
Time magazine’s most recent roundup of 200 inventions included an array of product types, spanning household gadgets and AI applications. Five distinct pharmaceuticals also made the list, not counting a shipping container for biologics and a vaccine for bees. Among the notable pharmaceutical advances on the list are a novel postpartum depression drug, a novel…
Prix Galien Awards: The most innovative biotech, pharma, and orphan drugs of 2023
Prix Galien names 2023 winners in pharma and biotech In the world of medical innovation, few accolades carry as much weight as the Prix Galien Awards, which highlight the advances in biotech, pharmaceuticals, and other domains. The 2023 winners include Bristol Myers Squibb’s Camzyos (mavacamten) as the best biotechnology product and Lilly’s Mounjaro (tirzepatide) and…
Biden names 31 tech hubs: Here are 10 relevant to pharma and biotech
Traditionally, the tech and biotech sectors in the U.S. have been concentrated in a handful of regions — most notably in areas such as Boston, Seattle, Silicon Valley and Southern California. But the Biden administration aims to distribute innovation more evenly through the U.S. To that end, the administration has designated 31 tech hubs across…
Guselkumab shows durable benefits in Crohn’s disease in LTE of phase 2 study
The interleukin-23 blocker guselkumab (Tremfya) continues to show promise in treating Crohn’s disease (CD). First winning FDA approval for plaque psoriasis in 2017, guselkumab recently demonstrated robust efficacy and a consistent safety profile in the long-term extension of the GALAXI Phase 2 study for CD. Some 54.1% of patients receiving guselkumab achieved clinical remission by…
Pfizer Ignite: Kathy Fernando’s vision for accelerating biotech innovation
Kathy Fernando, the senior vice president, head of Pfizer Ignite and Pfizer CentreOne, has had a professional trajectory marked by pivotal serendipities. One occurred when attending a seminar at the University of Pennsylvania, where she met Dr. Drew Weissman, a prominent immunologist and RNA vaccine researcher. Weissman, along with Katalin Karikó, recently received the Nobel…
The multi-billion dollar promise of efgartigimod and the broader FcRn inhibitor market
Thanks to promising candidates such as efgartigimod, the Fc receptor (FcRn) inhibitor market is poised to reshape the autoimmune disease treatment landscape. Sales projections for the market top $10 billion, as Driehaus Capital Management estimated in 2020. The addressable U.S. patient base spans more than 228,500 individuals across various conditions including myasthenia gravis, warm autoimmune…
Biotech sees new shoots of growth but has thorns left to prune
Today, Boston-based Ginkgo Bioworks’ recently unveiled a deal with Pfizer on RNA-based drug candidates, reflecting the growing interest in RNA therapies. Additionally, Carlsbad, California–based Ionis Pharmaceuticals inked a pact with Roche focused on RNA-targeted therapies for Alzheimer’s and Huntington’s diseases. This development underscores the resilience of prominent biotech hubs. According to data from real estate…
10 rising stars of North American biotech: Cities emerging as life sciences hubs
As the biotech industry matures, several North American cities are emerging biotech hubs, aiming to become the next epicenters of innovation and growth. While traditional hubs like Boston and San Francisco continue to dominate, a new wave of cities is making strides in biotech. For instance, Pittsburgh, an up-and-coming biotech hub, is tapping its biomanufacturing…
Sanofi ALTUVIIIO hemophilia A treatment: FDA accepts sBLA based on pediatric phase 3 data
The FDA has accepted Sanofi’s supplemental Biologics License Application (sBLA) for ALTUVIIIO, a novel, high-sustained factor VIII replacement therapy for adults and children with hemophilia A. The FDA based the decision on positive final data from the pivotal Phase 3 XTEND-Kids trial in children under 12 with hemophilia A. ALTUVIIIO (efanesoctocog alfa) is the first…
Cellares teams up with Bristol Myers Squibb to explore automated CAR-T cell therapy manufacturing
Less than a week after announcing that it has secured $255 million in Series C funding, South San Francisco-based startup Cellares has revealed that Bristol Myers Squibb has joined its Technology Adoption Partnership (TAP) program. To date, the company has raised more than $355 million in total financing. As part of the TAP program, Bristol…
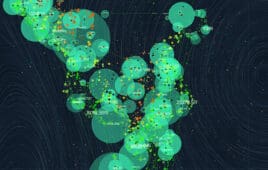
30 promising biotech startups: Scatter plot
The preceding visual representation of the biotech startups of 2023 are grouped — clustered — according to their focus areas. Each cluster color corresponds to a specific domain: Orange for advanced molecular techniques (Cluster 0). Blue for cell and gene therapies (Cluster 1). Green for AI-driven drug discovery (Cluster 2). Red for epigenetics and genomic…
Interactive snapshot: 30 promising biotech startups
2023 is shaping up to be a pivotal year for promising biotech startups. While the sector faced multiple waves of layoffs, a silver lining is evident: the growing maturity of AI and genomics technologies is fueling a new wave of startup advances, especially in drug development. Amidst the backdrop of that turbulence, a select group…
30 biotech startups making waves
The biotech industry is facing a reckoning in 2023. To date, roughly 100 biopharmas have cut workers this year, matching the total number of layoffs in the sector in 2022. Many biotech startups have been hit hard. The wave of job cuts comes on the heels of a biotech boom following the COVID-19 pandemic, when…