With the rise of biologics, drug delivery presents new challenges as many companies are still taking a fragmented approach, resulting in delayed (or suboptimal) product launches and life cycle management. The problem is that it’s extremely difficult, if not impossible, to fix drug delivery challenges after the fact, so companies need a clear strategy in advance.
Drug delivery may not sound sexy, but at least three factors – increased competition from biosimilars, formulation challenges of therapies in the pipeline, and an expansion of both disease areas and patients served by biologics – have come together to make it a critical issue for drug companies.
The Rise and Spread of Monoclonal Antibodies
Proteins like monoclonal antibodies (mAbs) are a rapidly growing segment of medicine, but they are too large to be administered orally, and require alternate delivery methods.
Many of these drugs in the pipeline have technical difficulties that will require new thinking on drug delivery. We believe that 15 to 20 percent of all proteins in development today have some kind of formulation challenge. As a result, drug companies must think about delivery early in the R&D process – even as early as molecular engineering – or they could wind up with a drug that receives FDA approval, yet isn’t commercially optimal. Moreover, as biopharma attempts to target new tissues with proteins in new ways (e.g., local targeting of the lung), formulation investments will need to be made early in the development cycle.
The Benefits of Novel Therapies
Biopharmaceutical companies that introduce a well-thought-out drug-delivery strategy should gain on multiple fronts with more patients, higher probability of hitting intended targets, better adherence to prescribed treatment regimens, and lower costs (see Figure).
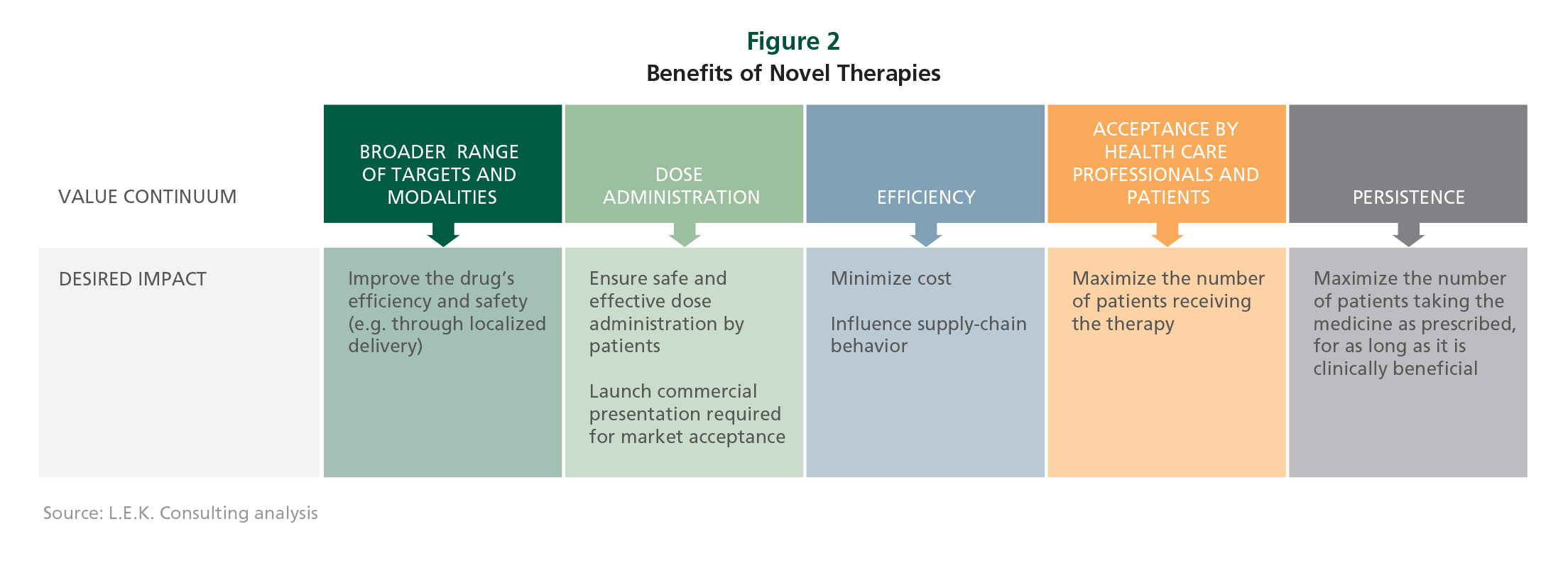 The increasing move toward self-administered therapies will heighten the importance of patient acceptance, adherence and compliance. Innovative designs could be as simple as offering an injectable drug in a premixed or preloaded syringe, or creating a formulation that requires less frequent injections.
The increasing move toward self-administered therapies will heighten the importance of patient acceptance, adherence and compliance. Innovative designs could be as simple as offering an injectable drug in a premixed or preloaded syringe, or creating a formulation that requires less frequent injections.
With legislation looming to promote biosimilars and increased pressure from both payers and patients to offer an increased value proposition, we believe innovation in drug delivery will become an increasingly important competitive differentiator. That occurred long ago with insulin, where there’s little therapeutic reason to choose one version of insulin over another, and devices have become a significant basis for competition. We believe something similar — if perhaps less extreme — is likely to evolve in areas where brands and biosimilars are proliferating.
A Drug Delivery Strategy
An L.E.K. survey of some 20 top global biopharmas put patient acceptance and persistence as the number one challenge that could be addressed by engaging customers with drug delivery technologies. But while these top executives realized they needed a drug-delivery strategy, few knew what to do to create one.
Here are some factors to consider:
Is a drug delivery strategy important to your organization? If you can answer yes to any of these questions, it is:
- Do you have self-administered biologics in highly-competitive markets?
- Do you have products used by patients with special requirements such as the elderly, frail, or children?
- Do you have biologics in your pipeline that have formulation challenges, such as lyophilization, concentration, shearing, or coagulation?
Will a one-off approach suffice or do you need a platform? The issue here is upfront cost vs. overall payoff.
Which technologies matter for the drugs in your pipeline? How do you ensure the strategy optimizes the needs across the portfolio?
Getting drug delivery right is crucial, but it won’t be easy. Those that can move to the forefront of new drug-delivery technologies with advanced formulations (such as nanoparticles and microspheres) or advanced devices (like microneedles and microinfusers) will be the ones that will win.
Filed Under: Drug Discovery




