Researchers from the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) are conducting a clinical trial on allergic reactions to mRNA-based COVID-19 vaccines. The single-site trial will enroll up to 100 people between 16 and 69 years old who had an allergic reaction to a first dose of COVID-19 mRNA vaccines. NIAID seeks participants…
J&J expands it long-acting injectables partnership with Midatech
Midatech Pharma (Nasdaq:MTP) announced today that it extended its R&D collaboration with Johnson & Johnson’s (NYSE:JNJ) Janssen Pharmaceuticals. Cardiff, United Kingdom-based Midatech, a drug delivery technology company focused on the biodelivery and biodistribution of medicines, initially entered into the R&D collaboration with Janssen on July 21, 2020. Get the full story at our sister site, Drug Delivery Business News.
MIT researchers come up with new strategy to create a potential antibiotic
Researchers at MIT are touting a novel method to synthesize a natural compound that has shown potential as an antibiotic. The chemists produced himastatin using the synthesis method and also managed to generate variants of the molecule, some of which showed antimicrobial activity, according to a news story from the university’s website. The compound appears…
Moderna skyrockets on Street-beating Q4 driven by COVID-19 vaccine sales
Moderna (NSDQ:MRNA) shares are on the rise on fourth-quarter results that came in ahead of the consensus forecast. MRNA shares were up 11.7% at $161.51 per share in midday trading today. MassDevice’s MedTech 100 Index — which includes stocks of the world’s largest medical device companies — was up 1.1%. The Cambridge, Massachusetts-based company posted…
PharmaJet partner touts interim safety results for needle-free COVID-19 vaccine
PharmaJet announced today that its partner, Technovalia, reported positive interim safety results from a needle-free COVID-19 vaccine trial. Golden, Colorado-based PharmaJet’s needle-free injection systems are being studied with Covigen, a DNA-based vaccine developed by French-Thai pharmaceutical company BioNet-Asia in collaboration with Melbourne, Australia-based Technovalia. Enrollment for the trial began in June 2021. Get the full story at…
Pfizer dips on Q4 revenue miss, provides 2022 guidance
Pfizer (NYSE:PFE) shares slid today on fourth-quarter results that came up shy of the consensus sales forecast. The pharmaceutical giant posted profits of $3.4 billion, or 59¢ per share, on sales of $23.8 billion for the three months ended Dec. 31, 2021, more than quadrupling its bottom-line on revenues that were more than doubled year-over-year.…
Medable and CVS Health partner to expand clinical trial access
Medable and CVS Health (NYSE:CVS) announced yesterday that they will collaborate to expand clinical trial access and engagement. Under the collaboration, Medable’s software platform will be combined with CVS Health’s community reach and experienced MinuteClinic providers to deliver clinical trials in a manner that improves patient access, engagement and retention. According to a news release,…
New AI out of MIT predicts how proteins will attach
MIT researchers are touting a machine learning model that can predict the complex that will form when proteins bind together. The technique represents an improvement on speed by somewhere between 80 and 500 times faster than state-of-the-art software methods and often predicts protein structures that are closer to actual structures that have been observed experimentally…
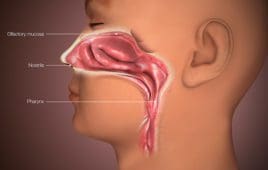
Could nasal vaccines be the next big weapon against COVID-19?
Because of the way they provide protection, nasal vaccines could be the best long-term way to prevent COVID-19 infection, according to experts cited in The New York Times. The Times reported that India-based Bharat Biotech, which has the Covaxin COVID-19 vaccine authorized in India and elsewhere, has an experimental COVID-19 nasal vaccine that may offer…
FDA fully approves Moderna’s COVID-19 vaccine
Moderna (NSDQ:MRNA) announced today that it received full FDA approval of the biologics license application for its COVID-19 vaccine. FDA’s approval for the company’s Spikevax mRNA COVID-19 vaccine covers the prevention of the virus in individuals aged 18 years or older. Moderna submitted for full FDA approval back in June 2021 and becomes the second…
BD boasts of a landmark advancement in flow cytometry
BD (NYSE:BDX) touted a study on innovation in flow cytometry that featured as the cover story of the journal Science. Franklin Lakes, New Jersey-based BD’s study was conducted in collaboration with the European Molecular Biology Laboratory (EMBL). It evaluated BD’s flow cytometry efforts that add fluorescence imaging and image-based decision-making to sort individual cells at high…
NeuroMetrix’s Quell wins FDA breakthrough designation to treat certain chemo side effects
NeuroMetrix (NSDQ:NURO) announced today that it received FDA breakthrough device designation for its Quell technology. Woburn, Massachusetts-based NeuroMetrix’s Quell garnered the breakthrough nod for reducing moderate-to-severe symptoms of chemotherapy-induced peripheral neuropathy (CIPN) that have persisted for at least six months following the end of chemotherapy. Get the full story at our sister site, MassDevice.
BD, Pfizer, Wellcome want to improve hospitals’ antimicrobial practices
BD (NYSE:BDX) announced yesterday that it will collaborate with Pfizer (NYSE:PFE) and Wellcome to advance antimicrobial stewardship practices. The collaboration builds on ongoing efforts to advance the role of diagnostics in addressing the challenge of antimicrobial resistance (AMR), according to a news release. The companies will survey existing diagnostic practices to highlight benefits and gaps in diagnostic testing in AMR…
Thermo Fisher Scientific completes $1.85B Peprotech acquisition
Thermo Fisher Scientific (NYSE:TMO) announced today that it completed its acquisition of PeproTech for approximately $1.85 billion. Cranbury, New Jersey-based PeproTech develops recombinant proteins, including cytokines and growth factors, for use in the development and manufacturing of cell and gene therapies and other broader cell culture applications. Waltham, Massachusetts-based Thermo Fisher said in a news…
FDA approves Bristol Myers Squibb drug to prevent graft versus host disease
The FDA today approved Bristol Myers Squibb’s Orencia (abatacept) for the prophylaxis (prevention) of acute graft versus host disease (aGVHD). According to an FDA release, this is the first FDA drug approval for aGVHD prevention, incorporating real-world evidence as a component of the determination of clinical effectiveness. Orencia — originally approved by the FDA in…
Thermo Fisher completes $17.4B acquisition of PPD
Thermo Fisher Scientific (NYSE:TMO) announced today that it completed its previously announced $17.4 billion acquisition of PPD. Waltham, Massachusetts-based Thermo Fisher announced in April that it would acquire the company that provides clinical research services to the biopharma and biotech industry. PPD ceased trading on the Nasdaq prior to opening today and will become part…
Gilead Sciences, Merck near the top of Newsweek’s most responsible companies list
A handful of big names in drug discovery and development are among the 500 “most responsible,” according to Newsweek. The outlet published its “America’s Most Responsible Companies 2022” list, marking the third installment of the compilation (in partnership with Statista), this time expanded to include 500 of the largest public corporations around. Companies were judged with an overall…
PerkinElmer launches new cloud-based platform for managing workflows
PerkinElmer (NYSE:PKI) announced today that it launched its PKeye workflow monitor for remotely managing and monitoring workflows. Waltham, Massachusetts–based PerkinElmer’s cloud-based PKeye workflow monitor enables laboratory personnel to remotely handle PerkinElmer instruments and workflows in real-time through 24/7 access and visibility into laboratory operations. Get the full story at our sister site, MassDevice.
BD expands biosciences business with reagent innovation center in San Diego
A life sciences real estate developer announced today that BD (NYSE:BDX) selected its property as a new facility for its biosciences business. Through a joint venture with Tishman Speyer and Bellco Capital, Breakthrough Properties confirmed that BD chose its Torrey View by Breakthrough property for an expanded San Diego reagent innovation center within its biosciences segment. Get…
Bruker acquires benchtop nuclear molecular imaging tech company Molecubes
Bruker (NSDQ:BRKR) announced today that it acquired Molecubes, a maker of benchtop preclinical nuclear molecular imaging (NMI) systems. Ghent, Belgium-based Bruker said in a news release that the acquisition, for which financial terms were not disclosed, will strengthen its position as a leading NMI solutions provider in preclinical and translational imaging research. The acquisitions will…
7 diabetes treatment innovations to look out for on World Diabetes Day
World Diabetes Day — Nov. 14 — centers around raising awareness for those with diabetes. This year, that aim remains the same, and medical technology companies continue to look for ways to continue improving the management of the metabolic disease. Some of those treatments involve insulin, which was discovered as a treatment for diabetes in…
Moderna takes big hit on missed Q3 projections
Moderna (NSDQ:MRNA) shares took a big hit today on third-quarter results that came up short of the consensus forecast. MRNA shares were down 17.1% at $286.80 per share in mid-morning trading today. MassDevice’s MedTech 100 Index — which includes stocks of the world’s largest medical device companies — was up 0.2%. Get the full story at our…
Pfizer to provide 50M COVID-19 vaccines for U.S. children
Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX) announced today that the U.S. bought 50 million more doses of its COVID-19 vaccine. The U.S. purchased the additional doses of the vaccine in its effort to support preparedness for pediatric vaccinations as it seeks authorization for use in younger adolescents and children. Earlier this month, Pfizer submitted a request…
DNA Script raises $165M Series C for DNA printing platform
DNA Script announced today that it raised $165 million in a Series C financing round, bringing its total capital raised to $280 million. South San Francisco-based DNA Script earmarked the funds raised to accelerate expansion and commercialization for the company’s Syntax benchtop nucleic acid printer and to broaden its portfolio of products powered by enzymatic…
Biogen misses on Q3 revenues, posts Street-beating EPS
Biogen (NSDQ:BIIB) shares ticked up today on third-quarter results that were mixed compared to the consensus forecast. The Cambridge, Massachusetts-based company posted profits of $329.2 million, or $2.22 per share, on sales of $2.2 billion for the three months ended Sept. 30, 2021, for a -53.1% bottom-line slide on a sales decline of -18%. Adjusted…