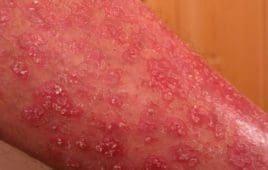
Novartis has entered into an exclusive license agreement with biotech companies Galapagos NV, Mechelen (Belgium) and MorphoSys AG, Planegg/Munich (Germany) regarding their compound MOR106. Under the agreement, Novartis acquires the exclusive global development and marketing rights to MOR106 for atopic dermatitis and all other potential indications. Novartis will make an upfront payment of EUR 95 million…
Novartis’s Cosentyx Goes Head-To-Head With Johnson & Johnson’s Tremfya
Novartis announced their plan to initiate ARROW, a head-to-head proof of concept study to assess the mechanistic superiority of the direct inhibition of IL-17A with Cosentyx (secukinumab) over the inhibition of IL-23 with Tremfya (guselkumab) in patients with psoriatic plaques resistant to treatment with Stelara[1]. Study results are expected in 2019. “We know there are different immune mechanisms…
Novartis Announces FDA Approval of Gilenya as the First Disease-Modifying Therapy for Pediatric Relapsing Multiple Sclerosis
Novartis Receives Second FDA Approval For Kymriah
Novartis announced the FDA has approved Kymriah (tisagenlecleucel) suspension for intravenous infusion for its second indication—the treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. Kymriah is not indicated…
New App Allows Patients to Participate in Ophthalmology Clinical Trials From Home
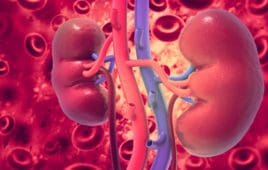
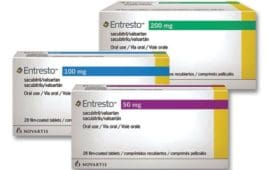
Entresto Helped Preserve Kidney Function in Patients With Chronic Heart Failure, Especially Those With Diabetes
Novartis Renews Commitment to Malaria Elimination
Study Shows Entresto Improves Physical, Social Activity in Heart Failure Patients
Novartis to Acquire AveXis for $8.7 Billion
Novartis Drug Approved By FDA To Treat Children With Rare Form Of Leukemia
The FDA expanded the indication for Tasigna (nilotinib) to include treatment of first- and second-line pediatric patients one year of age or older with Philadelphia chromosome-positive chronic myeloid leukemia in the chronic phase (Ph+ CML-CP). In the United States, Tasigna is now indicated for the treatment of adult and pediatric patients one year of age or older with newly…
Novartis Expands Alliance with Science 37 to Advance Virtual Clinical Trials Program
Novartis, Pear Therapeutics to Develop Digital Therapeutics for Patients With Schizophrenia, MS
Novartis’ Ultibro Breezhaler Improved Cardiac Function in COPD Patients
New Data Show Cosentyx Improved Quality of Life in Majority of Patients with Moderate to Severe Plaque Psoriasis
Novartis Forms Alliance to Develop Medicines for Treating Infectious Diarrheal Disease
FDA Approval of Glatopa as Generic Option for Relapsing MS
FDA OKs Cosentyx Label Update For Moderate To Severe Scalp Psoriasis
Novartis announced that the US Food and Drug Administration (FDA) has approved a label update for Cosentyx (secukinumab), the first interleukin-17A (IL-17A) antagonist approved to treat moderate to severe plaque psoriasis.1 The updated label includes Cosentyx data in moderate to severe scalp psoriasis—one of the difficult-to-treat forms of the disease, which affects approximately half of all psoriasis…
Novartis Receives FDA Approval for Cosentyx Label Update to Include Moderate-to-Severe Scalp Psoriasis
Novartis Completes Subsequent Offering Period of the Tender Offer for Advanced Accelerator Applications S.A.
Updated Analysis from Novartis’ ELIANA Trial
Outside U.S., Novartis Licenses First Ophthalmology Gene Therapy
Novartis Completes Tender Offer for Advanced Accelerator Applications
FDA Accepts Sandoz’s Submission for Proposed Biosimilar Adalimumab
Novartis Advances Head-to-head Superiority Trials of Cosentyx Versus Humira
Novartis Drug Promacta Receives FDA Breakthrough Therapy Designation For Use in SAA
Novartis today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy designation to Promacta (eltrombopag) for use in combination with standard immunosuppressive therapy for the treatment of patients with severe aplastic anemia (SAA) as a first-line therapy. Promacta, which is marketed as Revolade in most countries outside the US, is already…