Boehringer Ingelheim announced results from a phase III study, confirming that Cyltezo is equivalent to Humira, with no clinically meaningful differences in efficacy, safety, and immunogenicity in people with moderate-to-severe chronic plaque psoriasis.1 The 16-week data was presented at the European Association of Dermatology and Venereology Annual Meeting (EADV 2018) in Paris. “This phase III study builds on…
FDA Fast Track Designation Granted To Nintedanib For Treatment Of Systemic Sclerosis
The FDA has granted Fast Track designation to nintedanib for the treatment of systemic sclerosis with associated interstitial lung disease (SSc-ILD). The FDA’s Fast Track designation facilitates the development of new therapies that treat serious conditions and fulfill an unmet medical need in an effort to get treatments to those in need sooner. This designation…
Boehringer Ingelheim Gives Up On Alzheimer’s Drug
Boehringer Ingelheim refocuses PDE9 inhibition brain research on schizophrenia following results from Phase II Alzheimer’s trials. Boehringer Ingelheim announced that their Phase II Alzheimer’s disease (AD) trials with investigational compound BI 409306 had not met their efficacy endpoints and plans for further trials with BI 409306 in AD will therefore not be pursued. Instead, the company…
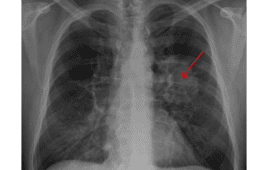
New Indication For Boehringer Ingelheim’s Gilotrif Okayed By FDA
FDA approves new indication for Gilotrif in EGFR mutation-positive NSCLC. The U.S. Food and Drug Administration (FDA) has approved a supplemental New Drug Application (sNDA) for Boehringer Ingelheim’s Gilotrif (afatinib) for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have non-resistant epidermal growth factor receptor (EGFR) mutations as detected by an…
Final Phase III Study Results Reinforces Safety and Efficacy of Praxbind
Boehringer Ingelheim Lung Disease Clinical Trial Moves Ahead
Orphan Drug Designation Granted for Treatment of Bone Marrow Disorders
FDA grants orphan drug designation to Boehringer Ingelheim’s investigational anti-CD33 monoclonal antibody BI 836858 for treatment of myelodysplastic syndromes. Boehringer Ingelheim today announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation to its anti-CD33 monoclonal antibody BI 836858 for the treatment of myelodysplastic syndromes (MDS). Orphan drug designation is granted…