 Bayer spin-off AiCuris (Wuppertal, Germany) has acquired exclusive rights to develop and commercialize RNA-based therapies for individuals with acute and chronic kidney diseases.
Bayer spin-off AiCuris (Wuppertal, Germany) has acquired exclusive rights to develop and commercialize RNA-based therapies for individuals with acute and chronic kidney diseases.
AiCuris will acquire the developmental therapies from the startup Hybridize Therapeutics (Leiden, Netherlands), which will get €100 million in upfront and milestone payments in addition to tiered royalties on net sales.
Sitting on Hybridize’s board of directors is Dr. John Maraganore, CEO of Alnylam Pharmaceuticals (NSDQ:ALNY).
At the heart of AiCuris’s licensing deal is an RNA-based antisense program for preventing BK virus infections in immunocompromised patients.
AiCuris will partner with Hybridize on preclinical development. AiCuris will take the lead on clinical development, which it anticipates will begin in two years.
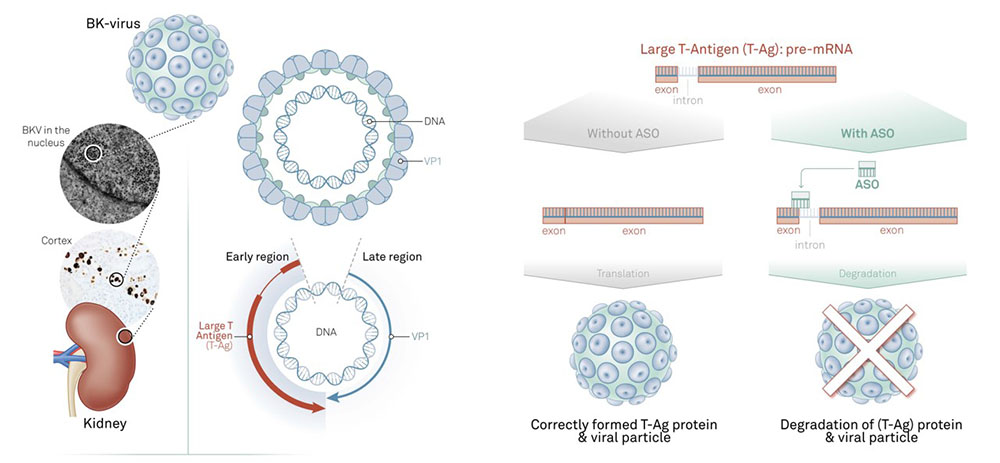
RNA antisense oligonucleotides mode of action. Image courtesy of Hybridize.
Reactivation of the BK virus (BKV) remains a significant challenge for patients undergoing kidney transplantation and can lead to a progressive graft dysfunction referred to as BK virus nephropathy (BKVN). BKNV can lead to allograft loss in kidney transplant recipients.
“We are excited to gain the rights to this exciting RNA-based antisense approach against BKV infections, further strengthening our anti-infective pipeline and building on our strong track record in the field of infectious diseases in transplant patients,” said Dr. Holger Zimmermann, CEO of AiCuris, in a news release.
Filed Under: clinical trials, Drug Discovery





Tell Us What You Think!
You must be logged in to post a comment.