[Photo by Daniel Schludi on Unsplash]
FDA recently granted emergency authorization to two SARS-CoV-2 vaccines and several other treatments.
Here are 11 of the most high-profile COVID-19 therapies to date. Some have already received emergency use authorization from FDA, while others will likely receive EUAs in 2021.
1. Tozinameran (BNT162b2) COVID-19 vaccine is the first to hit the U.S. market
A vial of the BNT162b2 COVID-19 vaccine. Image courtesy of Lisa Ferdinando from the Department of Defense.
Developers: Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX).
Description: Proposed indication is the prevention of COVID-19 in individuals 16 years of age and older. The vaccine uses an mRNA platform to targets the P2-mutated full spike protein of the SARS-COV-2 virus. The final analysis from a Phase 3 trial suggests that the vaccine is 95% effective at preventing COVID-19 symptoms and is generally well tolerated.
The vaccine’s current formulation must be stored between –80 and –60° C. Pfizer is currently evaluating whether the vaccine is stable at 2 to 8°C. The company is working on a new formulation that could be stable at room temperature.
Development status: Tozinameran became the first COVID-19 vaccine to win emergency use authorization from the FDA on Dec. 11. Pfizer plans to file a Biologics License Application (BLA) with FDA and anticipates full regulatory approval in 2021.
Pfizer intends to produce 1.3 billion doses of the vaccine by the end of 2021.
2. mRNA-1273 COVID-19 vaccine becomes second to win EUA
Developer: Moderna (NSDQ:MRNA)
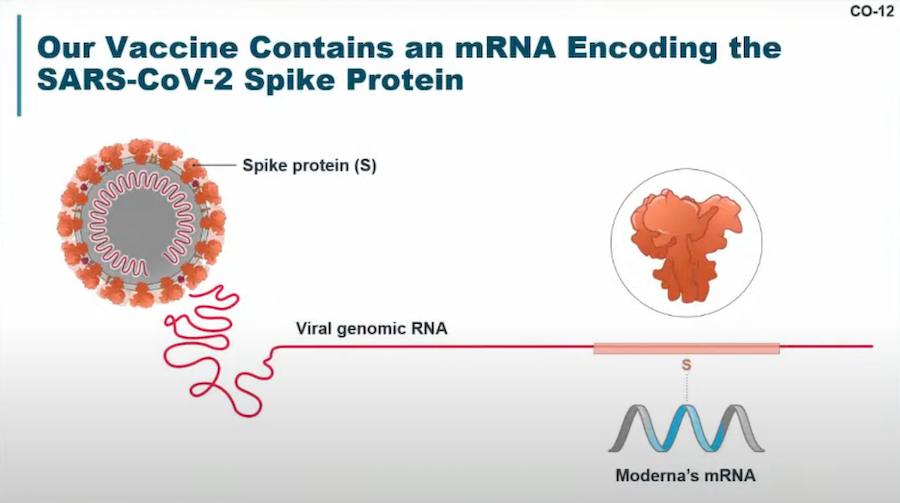
This image regarding the mRNA encoding of the SARS-CoV-2 spike protein was shared in the VRBPAC meeting. Image courtesy of Moderna.
Description: The vaccine uses a single mRNA sequence to encode the prefusion spike protein (S-2P) from the SARS-CoV-2 virus. Like the Pfizer-BioNTech vaccine, mRNA-1273 uses lipid nanoparticle technology to ferry mRNA to cells.
The Phase 3 clinical trial for the vaccine enrolled more than 30,000 U.S. participants, with 196 confirmed cases of COVID-19. The vaccine’s efficacy of 94.1% appears to be in line with the Pfizer-BioNTech vaccine, although the Moderna vaccine appeared appears to have a higher adverse-event rate.
Together with BNT162b2, mRNA-1273 sets a high bar for competition among COVID-19 vaccine developers but likely won’t shut out rivals in the near term.
Development status: FDA granted emergency use authorization to the vaccine on Dec. 18 following a positive assessment from the Vaccines and Related Biological Products Advisory Committee. Distribution of the vaccine began on Dec. 21.
3. Veklury (remdesivir): The first FDA-approved treatment for SARS-CoV-2
A ball-and-stick model of remdesivir. Image from Wikipedia.
Developer: Gilead (NSDQ: GILD)
Description: Remdesivir became one of the first COVID-19 therapies to receive emergency use authorization in May 2020. First developed to treat Ebola and Marburg virus infections, interest in the drug waned until the COVID-19 pandemic. Remdesivir became one of the first therapies to win emergency use authorization for SARS-CoV-2. While some reports suggest a modest clinical benefit for the treatment, critics such as the New York Times have called the drug “mediocre.” The organizers of a study known as the “Solidarity” trial concluded that remdesivir had “little or no effect on overall mortality, initiation of ventilation and duration of hospital stay in hospitalized [COVID-19] patients.”
Development status: Remdesivir obtained EUA in May 2020. The drug won full approval in Oct. 2020, making it the first FDA-approved treatment for COVID-19.
4. Casirivimab and imdevimab antibody cocktail wins EUA in November
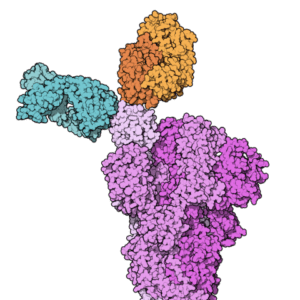
Space-filling model of the Fab fragments of the monoclonal antibodies casirivimab (REGN10933, blue) and imdevimab (REGN10987, orange) from Wikipedia.
Developer: Regeneron Pharmaceuticals (NSDQ:REGN)
Description: The cocktail has shown promise in treating patients with mild-to-moderate COVID-19 who face a high risk of developing severe infection or hospitalization. The IV-delivered therapy has shown promise in reducing hospitalizations and emergency room visits for patients at high risk for disease progression.
Regeneron claimed that the monoclonal antibody cocktail is the first treatment to show “statistically significant anti-viral activity” against COVID-19.
President Trump received the drug when hospitalized with COVID-19 in October, later describing it as a “cure.”
Development status: FDA allowed emergency use of the cocktail in Nov. 2020
5. Olumiant, a rheumatoid arthritis drug, shows COVID-19 promise

Winning an EUA for COVID-19 patients, Olumiant is primarily indicated for adults with moderately to severely active rheumatoid arthritis. Image courtesy of Eli Lilly.
Developer: Eli Lilly (NYSE:LLY)
Description: Olumiant (baricitinib), an anti-inflammatory medication, effectively reduces lung inflammation in COVID-19 patients when administered early after infection, according to a peer-reviewed study. FDA approved the Janus kinase inhibitor in 2018 for patients with moderate to severe rheumatoid arthritis.
The EUA for the product describes its use in “adult and pediatric patients 2 years and older with severe COVID-19 in combination with remdesivir.”
Development status: The product won emergency use in Nov. 2020. Lilly initially filed for an EUA to use Olumiant as a monotherapy but revised its application to use the drug with remdesivir.
6. Bamlanivimab wins EUA for patients at risk of severe COVID-19 infections
Developer: Eli Lilly (NYSE:LLY)
Description: Bamlanivimab (LY-CoV555) is a monoclonal antibody intended to treat mild-to-moderate COVID-19 in patients at risk of severe infections.
Development status: LY-CoV555 won an EUA from the FDA on Nov. 2020. In early December, Eli Lilly announced that it was launching a trial with UnitedHealth to test the antibody in high-risk patients.
HHS is working with Eli Lilly, its distributor AmerisourceBergen and local government health departments to distribute bamlanivimab. Image courtsey of the U.S. Department of Health and Human Services.
CVS Health (NYSE:CVS), the parent of CVS Pharmacy, is working with the U.S. government to administer the monoclonal antibody.
7. AstraZeneca aims to vaccinate much of the world with AZD1222
Developer: AstraZeneca (LON:AZN) and Oxford University
Description: AstraZeneca’s vaccine is less expensive than those from COVID-19 vaccine frontrunners Moderna and Pfizer/BioNTech, but the efficacy of ChAdOx1 nCoV-19 vaccine (AZD1222) is also lower. With an average efficacy of 70%, AZD1222 trails the mRNA vaccines from rivals by approximately 25%. For participants who received half of the initial dose of the two-dose vaccine, however, efficacy was 90%.
The Oxford-AstraZeneca vaccine was 62% effective when administered in two full doses, according to an interim analysis of a Phase 3 trial. Image courtesy of Oxford University.
In September, AstraZeneca announced that it was voluntarily pausing the Phase 3 trial so an independent committee could review safety data surrounding a single event of an unexplained illness that occurred in the U.K.
Development status: AstraZeneca has released interim results from its Phase 3 trial and published them in the Lancet.
The company says it can produce up to 3 billion vaccine doses in 2021.
AstraZeneca is partnering with the Gamaleya Research Institute, the developers of the Russian vaccine Sputnik V, to test a combination of vaccines to inoculate against SARS-CoV-2.
8. JNJ-78436725 vaccine could be offered in a single dose

A vial of the JNJ-78436725 vaccine candidate. [Image courtesy of Janssen Pharmaceuticals]
Description: The fourth vaccine in the U.S. to reach Phase 3 clinical trials, J&J’s Janssen Pharmaceuticals JNJ-78436725 vaccine could be the first single-dose vaccine to win an EUA from the FDA. Most of the COVID-19 vaccines in development require two. The company is, however, also testing a two-dose regimen for the vaccine in a Phase 3 trial.
The vaccine initially showed promise in inducing a neutralizing antibody response in rhesus macaques.
Development status: Johnson & Johnson is still testing the vaccine candidate in a Phase 3 trial, but the company aims to produce 1 billion doses of the vaccine by the end of 2021.
9. CD24Fc recombinant fusion protein
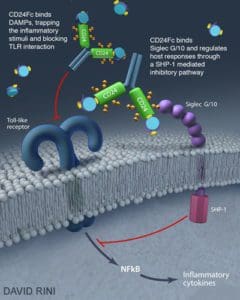
Image courtesy of David Rini/University of Maryland.
Developers: OncoImmune/Merck (NYSE:MRK)
Description: Researchers are testing the recombinant fusion protein CD24Fc in patients with severe COVID-19 infections requiring hospitalizations.
Containing a cell surface protein known as “CD24” and another from an antibody known as “Fc,” CD24Fc was an experimental drug from a privately-held biopharmaceutical company OncoImmune. Merck negotiated a deal to acquire the company and its CD24Fc therapy in November for $425 million.
Merck also has two COVID-19 vaccines in development, although they are in an earlier stage of development than those from top rivals.
Development status: The CD24Fc protein is undergoing testing in a Phase 3 trial focusing on severely ill hospitalized COVID-19 patients.
10. AZD7442 synthetic antibodies could offer long-lasting protection
Developer: AstraZeneca (LON:AZN)
Description: AZD7442 combines two monoclonal antibodies to home in specific SARS-CoV-2-produced antigens. The synthetic antibodies could offer protection for six months to a year, according to an AstraZeneca press release.
Development status: AZD7442 is undergoing a Phase 3 trial
11. VIR-7831
Developers: GlaxoSmithKline (LON: GSK) and Vir Biotechnology (NSDQ: VIR)
Description: VIR-7831 (GSK4182136) is an anti-SARS-CoV-2 antibody that could be used to treat mild-to-moderate COVID-19 infections. The antibody can achieve high concentrations in the lungs.
Development status: The two companies have launched a Phase 3 clinical trial to study the safety and efficacy of VIR-7831 to treat hospitalized adults with COVID-19. The study, known as ACTIV-3, is part of a series of trials from the NIH.
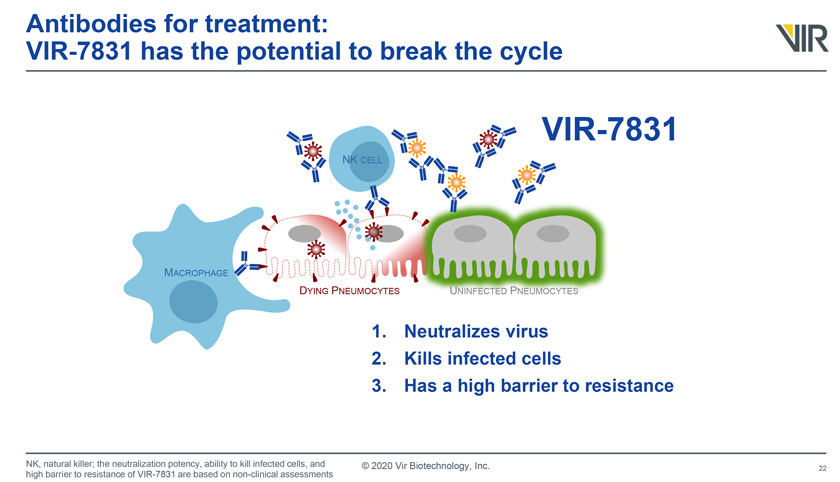
VIR-7831 image drawn from an SEC filing.
In October, GSK stated that interim Phase 3 results could be available before the end of the year and that it expected results from the primary endpoint of the trial in the first quarter of 2021.
Filed Under: clinical trials, Drug Discovery, Infectious Disease





Tell Us What You Think!
You must be logged in to post a comment.