[Zuranolone image courtesy of Wikipedia]
Here, we survey 10 antidepressants that may change the mental health landscape in the coming years.
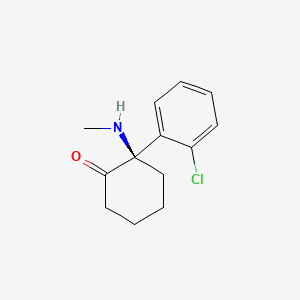
1. Esmethadone

[Image courtesy of Wikipedia]
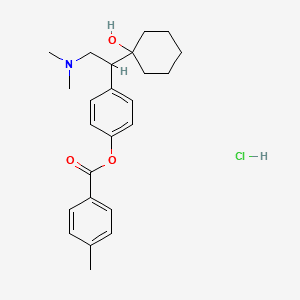
2. R-ketamine
[R-ketamine image courtesy of PubChem]
In general, ketamine also appears to have faster-acting effects than SSRI antidepressants.
Originated by Atai Life Sciences’ (Nasdaq:ATAI) subsidiary Perception Neuroscience, R-ketamine is also the focus of a collaborative program with Otsuka Pharmaceutical Co., Ltd., which is seeking to commercialize the drug candidate in Japan.
3. Ansofaxine hydrochloride
[Ansofaxine hydrochloride image courtesy of PubChem]
4. SEP-4199
A non-racemic ratio of amisulpride from Sumitomo Pharma subsidiary Sunovion could be a potential new therapy for bipolar depression. The company notes that SEP-4199 has a superior antidepressant activity to amisulpride. The developer claims that an 85:15 ratio of aramisulpride to esamisulpride offers an antidepressant benefit and reduces D2 receptor-related extrapyramidal side effects. The drug still retains D2 receptor binding-related benefits for bipolar disorder.
5. Psilocybin

[Psilocybin image courtesy of PubChem]
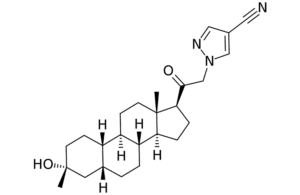
6. Zuranolone

[Zuranolone image courtesy of PubChem]
7. Seltorexant

[Seltorexant image courtesy of PubChem]
8. XEN1101
The differentiated Kv7 potassium channel modulator XEN1101 from Xenon Pharmaceuticals (Nasdaq:XENE) is the focus of the X-NOVA Phase 2 study focused on major depressive disorder. The company aims to complete the study in June 2023. Preclinical studies involving mice showed that the drug promoted the increased activity of KCNQ-type potassium channels, reversing depressive phenotypes related to chronic social defeat stress.
9. AXS-05
A combination of bupropion and dextromethorphan, AXS-05 is an oral, investigational NMDA receptor antagonist with multimodal activity that could potentially treat MDD, Alzheimer’s disease agitation and smoking cessation. Developed by Axsome Therapeutics (Nasdaq:AXSM), AXS-05 consists of a proprietary formulation and dose of dextromethorphan (DM) and bupropion. Earlier this year, Axsome announced the publication results from the pivotal ASCEND Phase 2 clinical trial of AXS-05 in MDD in the American Journal of Psychiatry. The study found that the drug candidate significantly improved depressive symptoms compared with bupropion, also known as Wellbutrin.
10. NRX-101
Developed by NRx Pharmaceuticals (Nasdaq:NRXP), Cyclurad (NRX-101) is a combination of the antibiotic D-cycloserine and lurasidone, an atypical antipsychotic under development for bipolar depression. The company believes D-cycloserine inhibits the brain’s NMDA receptor and raises glutamate/glutamine levels in the anterior cingulate cortex. NRx is testing the drug candidate in a Phase 2 study in bipolar depression in patients with sub-acute suicidal ideation and behavior. A separate Phase 3 study tests the combination of NRX-100 (ketamine HCl) and NRX-101 in patients with severe bipolar depression with acute suicidal ideation and behavior. NRX-101 has won Fast Track and Breakthrough Therapy Designation from FDA.
Filed Under: clinical trials, Drug Discovery, Psychiatric/psychotropic drugs





Tell Us What You Think!
You must be logged in to post a comment.